Radiation Therapy (Prostate Cancer)
Definition
Two different approaches to prostate radiotherapy exist, and both produce the same changes. In brachytherapy, interstitial radiation is delivered by implanted seeds with a radioisotope (131 I,192 Ir, or198 Au). In external beam therapy, a beam delivers radiation to the whole pelvis with a boost to the prostate.
Epidemiology
Etiology
The early published literature on the pathology of irradiated prostate cancer is based on external beam radiation.
Clinical Features
Serum prostate-specific antigen (PSA) and a rectal examination may suggest whether or not radiotherapy has eradicated all the tumor.2
Differential diagnosis
The benign prostate shows acinar atrophy, with acinar distortion and decreased number and size. In the most extreme form, only single cells may remain. Cytoplasm is usually decreased. Some nuclei are pyknotic, while others may be enlarged, and atypical, exceeding the atypia of cancer. Basal cell hyperplasia or squamous metaplasia may exist.
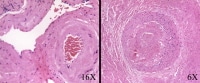
In both benign and malignant prostate, stromal fibrosis may occur, and vascular changes include myointimal proliferation, luminal narrowing, and foamy macrophages. The hyalinized and thickened vessels (see Image 11) provide a clue that radiation therapy is in the history, even if not specified by the clinician.
Histologic features of irradiated cancer and reactive prostate, however, can overlap regardless of the form of radiotherapy. Thus calling some cases indeterminate or atypical suspicious acinar proliferation (ASAP), a designation applied to most of the 20% non-negative biopsies in one study, is reasonable.3
Histologic features of irradiated cancer and reactive prostate, however, can overlap regardless of the form of radiotherapy. Thus calling some cases indeterminate or atypical suspicious acinar proliferation (ASAP), a designation applied to most of the 20% non-negative biopsies in one study, is reasonable.3
Gross Findings
No findings are specific to radiotherapy.
Microscopic Findings
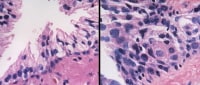
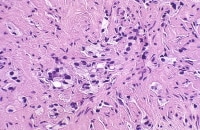
In the non-neoplastic irradiated prostate, nuclear enlargement and smudged chromatin are the most notable changes.
In basal cells, normally a single layer (left), nuclear enlargement can be found, and they may become so hyperplastic that they form several layers and secretory cells are inconspicuous (right). Irregular, potato-shaped nuclei are pathognomonic for basal cells.
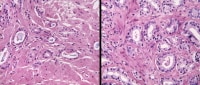
Identification of irradiated cancer is a problematic area in pathology, now that increased numbers of post-treatment biopsies are being performed. Early changes include cytomegaly, vacuoles, and nucleomegaly with persistent single and occasionally double nucleoli in each nucleus (left). Later changes include atrophy and sometimes cytoplasmic vacuolation, with the nucleoli now being inconspicuous.
In this matched set of photomicrographs from the same patient, compared with pre-treatment grade 3 cancer (left), the main post-treatment change is atrophy (right). Note, however, the maintenance of infiltrative pattern, angulated acini, absence of basal cells, and inspissated luminal blue mucin characteristic of cancer. Depending on the duration of irradiation, one may see all atrophic cancer acini, unchanged acini, or a combination of atrophic and unchanged acini.
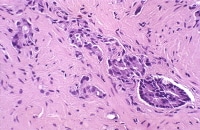
Needle biopsy with postirradiation grade 3 cancer (left), a focus of high-grade prostatic intraepithelial neoplasia (PIN; center), and grade 4 cancer (right).
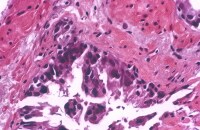
The residual neoplasm loses architectural differentiation while retaining the cytologic features of cancer. The acini are decreased in number and smaller in size, with a haphazard arrangement.
In the grade 4 component of cancer, the acinar luminal structure breaks down; overgrading the apparent single cells as grade 5 is a temptation. The nucleoli have disappeared, indicating maximal effect. Some cases have increased Paneth cells (Siders, 1992).
In this biopsy, high-grade prostatic intraepithelial neoplasia (PIN) with radiation effect is a helpful feature that should prompt a search for cancer. However, the frequency of PIN in irradiated prostate cancer (based on salvage prostatectomy findings) is decreased to 60% of cases (Cheng, 1998). In contrast, 82% of nonirradiated, step-sectioned prostates show high grade PIN (McNeal, 1986).
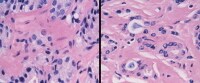
In cases in which the history of irradiation is not given or is uncertain, stromal and vascular changes can cue the pathologist to recognize radiation effect. The stroma becomes fibrotic, and the cellularity of normal vessel walls (normal: left) increases because of smooth muscle proliferation (right).
Immunohistochemistry
Benign acini with no residual cancer are positive for high molecular weight basal cell cytokeratin 34ßE12, prostate-specific antigen (PSA), and prostatic acid phosphatase (PAP). The absence of basal cell cytokeratin staining is most reliable in diagnosing residual cancer, provided good internal positive control exists. Ways of optimizing the intensity and percent of non-neoplastic epithelium staining with this marker have been devised. These methods involve steam heat and ethylenediaminetetraacetic acid (EDTA) for antigen retrieval.4
Yang et al tested the new marker α-methylacyl-CoA racemase (AMACR, P504S) to determine its usefulness in diagnosing postirradiation cancer. It was consistently reactive in 28 cancers and nonreactive in 12 benign postbiopsy cases.5 However, used in conjunction with cytokeratin 34ßE12, P504S was not considered to increase recognition of postirradiation cancer compared with cytokeratin 34ßE12 alone.6
If cancer cells are present, their viability can be assessed by positive staining with pan-cytokeratin and PSA.7 In positive biopsies 12 months after radiotherapy, PCNA staining is useful. A negative result predicts resolution of tumor in 83-97% of cases by 36 months. Positive PCNA correlates with local failure in 49-79% of cases, but when PCNA is present in an early biopsy (12-18 months), PCNA may still disappear.8
Preradiation expression of MIB-19 and p5310 predicts postradiation recurrence. Increased p53 expression occurs after radiation, indicating that cells with abnormal overexpression were protected from cell death. p53 expression in radioresistant cancer (postradiation) is less likely if patients get neoadjuvant hormonal treatment.11 No significant changes in bcl-2 or p21WAF1 are found.
Prognosis and Predictive Factors
Postradiotherapy grading of cancer was not endorsed by the International Consultation Meeting on Prostatic Intraepithelial Neoplasia & Pathologic Staging of Prostatic Carcinoma, November 1995. This issue remains controversial, with conflicting data.12 Cancer Gleason score in needle biopsies in nonirradiated prostates matches the score by ±1 in the prostatectomy 74% of the time.13 A Mayo Clinic study evaluating patients treated with radiation and salvage prostatectomy showed a mean Gleason score of 6.2 in biopsy and 6.8 in prostatectomy.14
However, other studies show posttherapy upgrading. A 24% increase in the number of poorly differentiated (score 8-10) tumors after treatment was found.15,14 The strongest argument for grading is that by multivariate analysis, only Gleason grade predicts cancer-specific survival. A decrease in positive margins was also found.14 Patients whose tumors had dedifferentiated had poorer survival than those patients who retained their original tumor grade. This was interpreted as time-dependent progression.16
The recommended timing of a prostate biopsy after irradiation is a one-year minimum after completion of radiation therapy. This approach reliably discloses residual cancer.17 The number of biopsy sites should be as many as possible. Ultrasound-guided biopsies beginning 12 months after radiation therapy revealed failure in 103 of 479 (21%) of patients.18 Sixty-seven of these patients converted to negative biopsies after a mean 28 months.Thus, the tumor may take up to 28 months to resolve. In another study of 160 patients, at a median 6.7-year follow-up, 21% of patients had a positive biopsy result.19 The radiation therapy failure rate is 25-90%, depending on whether failure is based on biochemical or biopsy evidence. Notably, however, 17% of patients with a positive biopsy remain clinically free of disease at greater than 10 years of follow-up.20 In patients undergoing salvage radical prostatectomy after failure, recurrence was detected by biopsy after a mean 3.5 (0.5-17) years.21
With stage II prostate cancer, 95% of postradiation biopsies were found negative for cancer, while this figure was 55% for stage III cancer.22
Salvage prostatectomy after radiation failure produced a 5-year, cancer-specific survival of 91%; 83% of patients were free of metastases.
Multimedia
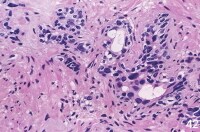 | Media file 1: In the non-neoplastic irradiated prostate, nuclear enlargement and smudged chromatin are the most notable changes. |
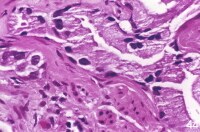 | Media file 2: This nuclear enlargement may be extreme, but no prominent secretory cell nucleoli exist. |
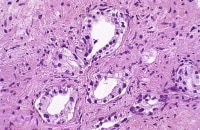 | Media file 3: The preservation of at least a focal basal cell layer is a key finding (upper left). |
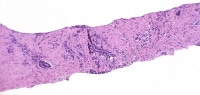 | Media file 7: Needle biopsy with postirradiation grade 3 cancer (left), a focus of high-grade prostatic intraepithelial neoplasia (PIN; center), and grade 4 cancer (right). |
Keywords
radiation therapy, prostate cancer, cancer, radiation, prostate, prostate radiotherapy, brachytherapy, external beam therapy, radioisotope, radiation therapies, external beam therapies, cancer of the prostate





0 comments:
Post a Comment