Refractory Cytopenia With Unilineage Dysplasia
Overview
Myelodysplastic syndromes (MDS), as defined by the World Health Organization (WHO), represent clonal hematopoietic stem cell disorders resulting in ineffective hematopoiesis. Refractory cytopenia with unilineage dysplasia (RCUD) is a category of MDS characterized by morphologic dysplasia of a single myeloid lineage with associated peripheral blood cytopenia. As with all types of MDS, there is a risk of progression or transformation to acute myeloid leukemia (AML). However, RCUD is considered a low-risk MDS; cases follow a prolonged and sometimes indolent clinical course.
Disorders
Refractory neutropenia with unilineage dysplasia
Refractory anemia with unilineage dysplasia
Refractory thrombocytopenia with unilineage dysplasia
Refractory anemia with unilineage dysplasia
Refractory thrombocytopenia with unilineage dysplasia
Definition
RCUD represents a heterogeneous group of MDS characterized by morphologic dysplasia of a single myeloid lineage with associated peripheral blood cytopenia. This category of MDS includes refractory anemia (RA), refractory neutropenia (RN), and refractory thrombocytopenia (RT); specific diagnoses are made on the basis of the morphologically abnormal lineage present in the bone marrow.1 Although the dysplastic lineage most commonly corresponds to the identified cytopenia, discordant findings (eg, neutropenia with megakaryocyte dysplasia) do not preclude the diagnosis of RCUD. Cases with bicytopenias and unilineage dysplasia are also included in this group; data suggest that such features portend a worse prognosis.2
The WHO, adopting criteria from the Myelodysplastic Working Group, has defined thresholds for cytopenias (ie, hemoglobin level <10 g/dL, absolute neutrophil count (ANC) <1.8 x 103/μL, and platelet count <100 x 103/μL), although morphologic and cytogenetic findings alone are considered diagnostic. In the bone marrow, at least 10% of a given myeloid lineage should be affected. Cases demonstrating Auer rods and/or increased numbers of myeloblasts (>5% in bone marrow) are not included in this group (see Refractory Anemia with Excess Blasts). As with all forms of MDS, other causes of myeloid dyspoiesis should be excluded before assigning this diagnosis.
The WHO, adopting criteria from the Myelodysplastic Working Group, has defined thresholds for cytopenias (ie, hemoglobin level <10 g/dL, absolute neutrophil count (ANC) <1.8 x 103/μL, and platelet count <100 x 103/μL), although morphologic and cytogenetic findings alone are considered diagnostic. In the bone marrow, at least 10% of a given myeloid lineage should be affected. Cases demonstrating Auer rods and/or increased numbers of myeloblasts (>5% in bone marrow) are not included in this group (see Refractory Anemia with Excess Blasts). As with all forms of MDS, other causes of myeloid dyspoiesis should be excluded before assigning this diagnosis.
Epidemiology
The epidemiologic features of RCUD are similar to those of most types of MDS. The average age of patients at presentation is approximately 65-70 years. In contrast with other forms of MDS, there is no gender predilection.3Exposure to agricultural chemicals and a history of smoking confer an increased risk of the development of RA andrefractory anemia with ring sideroblasts (RARS).4 The precise incidence and prevalence of RCUD have not been well established; however, RA represents the majority of cases of RCUD.
Clinical Features
Clinical features are often nonspecific and are generally related to the corresponding cytopenia (ie, bleeding associated with thrombocytopenia; fatigue and exercise intolerance associated with anemia). In many cases, the patient is asymptomatic, and cytopenia is identified during routine evaluation.
Morphologic Features
The morphologic features of the peripheral blood and bone marrow are currently the gold standard for the diagnosis of MDS.
Peripheral blood
Evaluation of the peripheral blood smear demonstrates 1 or more cytopenias. When affected, the erythroid lineage is characterized by a macrocytic or normocytic anemia with variation in red cell size and shape (anisopoikilocytosis). Platelets may vary in size; giant forms may be present. Dysplasia of the granulocyte lineage may be assessed on the peripheral blood smear; hypolobated nuclei and hypogranularity are the most common features.
Bone marrow
The bone marrow is normocellular to hypercellular; at least 10% of a lineage demonstrates dysplastic features. In addition to nuclear and cytoplasmic atypia, the erythroid series may show megaloblastoid features (ie, dyssynchronous maturation of nucleus and cytoplasm and/or disproportionately large cell size relative to the corresponding normal stage of development); such megaloblastoid features are indistinguishable from those seen in vitamin B12 or folate deficiency. Ring sideroblasts may be present; however, if they account for fewer than 15% of erythroid precursors, a diagnosis of refractory anemia with ring sideroblasts (RARS) is more appropriate.
The morphologic features of erythroid, granulocytic, and megakaryocytic dysplasia are presented below (see Table 1 and Media files 1-7).
Peripheral blood
Evaluation of the peripheral blood smear demonstrates 1 or more cytopenias. When affected, the erythroid lineage is characterized by a macrocytic or normocytic anemia with variation in red cell size and shape (anisopoikilocytosis). Platelets may vary in size; giant forms may be present. Dysplasia of the granulocyte lineage may be assessed on the peripheral blood smear; hypolobated nuclei and hypogranularity are the most common features.
Bone marrow
The bone marrow is normocellular to hypercellular; at least 10% of a lineage demonstrates dysplastic features. In addition to nuclear and cytoplasmic atypia, the erythroid series may show megaloblastoid features (ie, dyssynchronous maturation of nucleus and cytoplasm and/or disproportionately large cell size relative to the corresponding normal stage of development); such megaloblastoid features are indistinguishable from those seen in vitamin B12 or folate deficiency. Ring sideroblasts may be present; however, if they account for fewer than 15% of erythroid precursors, a diagnosis of refractory anemia with ring sideroblasts (RARS) is more appropriate.
The morphologic features of erythroid, granulocytic, and megakaryocytic dysplasia are presented below (see Table 1 and Media files 1-7).
The presence of hypoplasia does not exclude a diagnosis of RCUD, because hypoplastic forms are not uncommon, accounting for approximately 10% of confirmed cases of MDS. Importantly, morphologic changes identical to those of dysplasia may occur as an artifact of poor preservation or as a result of inadequate storage during transport of samples; extra caution is advised when evaluating suboptimal specimens.
Table 1. Morphologic Features of Refractory Cytopenia With Unilineage Dysplasia (RCUD)
Open table in new window
Immunophenotypic Features and Methods
Immunohistochemical studies are not necessary in most cases of RCUD. However, staining for CD34, a relatively specific hematopoietic stem cell marker in bone marrow, may be used to localize immature precursors and to exclude higher-grade forms of MDS.5 Importantly, CD34 is not lineage specific, and some myeloblast populations do not express CD34; interpretation of staining patterns should not be performed in isolation.
The use of flow cytometry in the diagnosis of myelodysplasia has progressively evolved from identifying myeloblast populations to recognizing aberrant immunophenotypic patterns of myeloid and erythroid maturation. Because the diagnosis of MDS is complicated by the lack of morphologic specificity, there is an ever-increasing body of literature supporting the application of flow cytometry to fill diagnostic gaps.6 Aberrant antigenic patterns may be identified on the blast populations, including expression of CD7 (a T-cell–associated antigen) and/or CD56 (a natural killer (NK)-cell–associated antigen). Myeloid maturation patterns may also be assessed; evidence suggests that a limited panel of markers, including glycophorin A, CD71 (transferrin receptor), CD105 (endoglin), cytosolic ferritin subunits, and mitochondrial ferritin, may be used to accurately assess for erythroid dysplasia and to identify ringed sideroblasts.7,8
The use of flow cytometry in the diagnosis of myelodysplasia has progressively evolved from identifying myeloblast populations to recognizing aberrant immunophenotypic patterns of myeloid and erythroid maturation. Because the diagnosis of MDS is complicated by the lack of morphologic specificity, there is an ever-increasing body of literature supporting the application of flow cytometry to fill diagnostic gaps.6 Aberrant antigenic patterns may be identified on the blast populations, including expression of CD7 (a T-cell–associated antigen) and/or CD56 (a natural killer (NK)-cell–associated antigen). Myeloid maturation patterns may also be assessed; evidence suggests that a limited panel of markers, including glycophorin A, CD71 (transferrin receptor), CD105 (endoglin), cytosolic ferritin subunits, and mitochondrial ferritin, may be used to accurately assess for erythroid dysplasia and to identify ringed sideroblasts.7,8
Molecular/Genetic Features and Methods
Approximately one half of cases of RA have a demonstratable cytogenetic abnormality, most often involving chromosomes 5 or 7.3 Although abnormalities in RA are limited to a small number of chromosomes, proper characterization is prognostically significant.9 Too few cases of RN or RT have been reported to definitively establish cytogenetic associations.
Prognosis and Predictive Factors
RCUD is considered a low-grade form of MDS; the median survival is prolonged—on the order of 5 years.3 There is some disagreement as to the likelihood of the subsequent development of acute myeloid leukemia, although the risk appears to be low (1-2%).3,10
The widely accepted International Prognostic Scoring System (IPSS) was introduced by the Myelodysplastic Syndrome Working Group in 1997. The IPSS stresses the importance of cytogenetic findings. It has been successfully used to stratify patients on the basis of the percentage of bone marrow myeloblasts; karyotypic abnormality; and cytopenia.9 Incorporating associated laboratory findings, the classification system separates patients into 4 risk groups, which represent an increasing likelihood of progression to acute leukemia and decreasing survival (see Table 2). The majority of patients with a WHO diagnosis of RCUD fall into the low and intermediate-1 risk groups. The prognostic value of a recently proposed WHO Prognostic Scoring System (WPSS), which considers the impact of transfusion requirements and morphologic classification, has also been established.11
Table 2. International Prognostic Scoring System (IPSS)*
The widely accepted International Prognostic Scoring System (IPSS) was introduced by the Myelodysplastic Syndrome Working Group in 1997. The IPSS stresses the importance of cytogenetic findings. It has been successfully used to stratify patients on the basis of the percentage of bone marrow myeloblasts; karyotypic abnormality; and cytopenia.9 Incorporating associated laboratory findings, the classification system separates patients into 4 risk groups, which represent an increasing likelihood of progression to acute leukemia and decreasing survival (see Table 2). The majority of patients with a WHO diagnosis of RCUD fall into the low and intermediate-1 risk groups. The prognostic value of a recently proposed WHO Prognostic Scoring System (WPSS), which considers the impact of transfusion requirements and morphologic classification, has also been established.11
Table 2. International Prognostic Scoring System (IPSS)*
Open table in new window
| Prognostic Variables | 0 | 0.5 | Score 1 | 1.5 | 2 |
| Bone marrow blasts (%) | <5% | 5-10% | 11-19% | 20-30% | |
| Karyotypeΐ | Good | Intermediate | Poor | ||
| Cytopenias | 0-1 | 2-3 |
Open table in new window
| Risk Group | Cumulative Score | Approximate Median survival (years) |
| Low risk | 0 | 6 |
| Intermediate – 1 | 0.5-1 | 3.5 |
| Intermediate – 2 | 1.5-2 | 1 |
| High risk | >2.5 | 0.5 |
*Adapted from Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. Mar 15 1997;89(6):2079-88.ΐ Karyotype: Good: normal, -Y, del(5q), del(20q); Poor: complex (>3 abnormalities) or chromosome 7 abnormalities; Intermediate: all other abnormalities.
DIFFERENTIALS
| Acute Myelogenous Leukemia | Sideroblastic Anemias |
| Aplastic Anemia | Toxic (drug, alcohol) |
| Myelodysplastic Syndrome | |
| Neutropenia | |
| Nutritional Deficiency (vitamin B12, folate) |
Multimedia
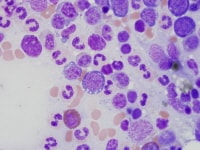 | Media file 1: Erythroid dysplasia: cytoplasmic vacuolization. |
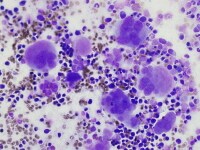 | Media file 2: Megakaryocyte dysplasia: hyperlobate nuclei and megakaryocyte aggregates. |
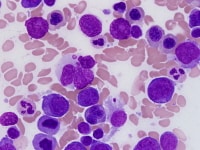 | Media file 3: Erythroid precursor with nuclear atypia. |
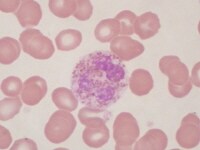 | Media file 4: Neutrophil with abnormal cytoplasmic granules. |
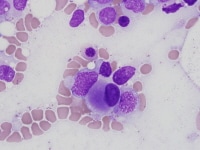 | Media file 5: Micromegakaryocyte. |
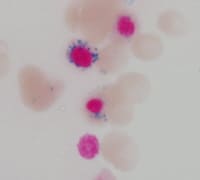 | Media file 6: Ring sideroblasts representing fewer than 15% of nucleated erythroid precursors. |
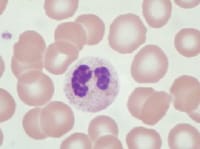 | Media file 7: Pseudo–Pelger-Huet cell: neutrophil with bilobed nucleus. |
Keywords
refractory cytopenia, refractory anemia, refractory thrombocytopenia, refractory neutropenia, myelodysplasia, myelodysplastic syndromes, MDS





0 comments:
Post a Comment