Sex Cord-Stromal Tumors
Definition;
Sex cord-stromal tumors are groups of tumors composed of granulosa cells, theca cells, Sertoli cells, Leydig cells, and fibroblasts of stromal origin, singly or in various combinations.1 According to the WHO, sex cord-stromal tumors are classified into the following categories:
- Granulosa-stromal cell tumors
- Granulosa cell tumor group
- Adult
- Juvenile
- Thecoma-fibroma group
- Thecoma, not otherwise specified
- Typical
- Luteinized
- Fibroma
- Cellular fibroma
- Fibrosarcoma
- Stromal tumor with minor sex cord elements
- Sclerosing stromal tumor
- Signet ring cell stromal tumor
- Unclassified (fibrothecoma)
- Thecoma, not otherwise specified
- Granulosa cell tumor group
- Sertoli-stromal cell tumors
- Sertoli-Leydig cell tumors group (androblastomas)
- Well differentiated
- Of intermediate differentiation
- Variant with heterologous elements
- Poorly differentiated (sarcomatoid)
- Variant with heterologous elements
- Retiform
- Variant with heterologous elements
- Sertoli cell tumors
- Stromal-Leydig cell tumor
- Sertoli-Leydig cell tumors group (androblastomas)
- Sex cord-stromal tumors of mixed or unclassified cell types
- Sex cord tumor with annular tubules
- Gynandroblastoma
- Sex cord-stromal tumor, unclassified
- Steroid cell tumors
- Stromal luteoma
- Leydig cell tumor group
- Hilus cell tumor
- Leydig cell tumors nonhilar type
- Leydig cell tumors, not otherwise specified
- Steroid cell tumors, not otherwise specified
- Well differentiated
- Malignant
Granulosa cell tumor, microfollicular pattern. Call-Exner bodies are formed by a discrete array of granulosa cells with angular grooved nuclei and central inspissated eosinophilic material (H&E, x400).
Epidemiology
Sex cord-stromal tumors account for approximately 8% of all ovarian tumors.
Immunohistochemistry
Although various markers have been reported to stain sex cord-stromal tumors (eg, CD99, CD56, A103, müllerian inhibiting factor, vimentin), inhibin and calretinin have proven to be the most helpful to date. These tumors can also be positive for cytokeratin (CAM 5.2, AE1/AE3), CD10, ER, PR, smooth muscle actin, desmin, S100 protein, and WT-1.2,3,4,5,6,7,8,9,10,11,12,13,14
CD56 is a sensitive marker of ovarian sex cord-stromal tumors and may also be useful in the diagnosis of this group of neoplasms, especially in cases that are inhibin or calretinin negative, and the differential diagnosis includes neoplasms that are CD56 negative.
Although various markers have been reported to stain sex cord-stromal tumors (eg, CD99, CD56, A103, müllerian inhibiting factor, vimentin), inhibin and calretinin have proven to be the most helpful to date. These tumors can also be positive for cytokeratin (CAM 5.2, AE1/AE3), CD10, ER, PR, smooth muscle actin, desmin, S100 protein, and WT-1.2,3,4,5,6,7,8,9,10,11,12,13,14
CD56 is a sensitive marker of ovarian sex cord-stromal tumors and may also be useful in the diagnosis of this group of neoplasms, especially in cases that are inhibin or calretinin negative, and the differential diagnosis includes neoplasms that are CD56 negative.
Tumor spread and staging
The International Federation of Gynecology and Obstetrics (FIGO) staging of ovarian tumors is as follows:15
- I. Growth limited to the ovaries
- A: Growth limited to one ovary; no ascites. No tumor on the external surface; capsule intact.
- B: Growth limited to both ovaries; no ascites. No tumor on the external surface; capsule intact.
- C: Tumor either Stage IA or Stage IB, but with tumor on the surface of one or both ovaries, or with capsule ruptured, or with ascites containing malignant cells or with positive peritoneal washings.
- II. Growth involving one or both ovaries with pelvic extension
- A: Extension and/or metastases to the uterus and/or tubes
- B: Extension to other pelvic tissues
- C: Tumor is either Stage IIA or IIB but with tumor on the surface of one or both ovaries; or with capsule ruptured; or ascites present containing malignant cells or with positive peritoneal washings.
- III. Tumor involving one or both ovaries with peritoneal implants outside the pelvis and/or positive retroperitoneal or inguinal nodes. Superficial liver metastasis equals Stage III.
- A. Tumor grossly limited to the true pelvis with negative nodes but with histologically confirmed microscopic seeding of abdominal peritoneal surfaces.
- B. Tumor of one or both ovaries with histologically confirmed implants of abdominal peritoneal surfaces, none exceeding 2 cm in diameter. Nodes are negative.
- C. Abdominal implants more than 2 cm in diameter and/or positive retroperitoneal or inguinal nodes.
- IV. Growth involving one or both ovaries with distant metastasis.
If pleural effusion is present, positive cytology must be present to allot a case to stage IV. Parenchymal liver metastasis equals stage IV.
Granulosa Cell Tumor Group
Definition
The WHO defines granulosa cell tumor (GCT) as a neoplasm composed of a pure or at the least a 10% population of granulosa cells, often in a fibroatheromatous background. Two major subtypes are recognized, an adult and a juvenile type.1
EpidemiologyGranulosa cell tumors account for about 1.5% of all ovarian tumors and occur in a wide age range, from newborn to postmenopausal women.16
Adult Granulosa Cell Tumor
EpidemiologyAdult granulosa cell tumor accounts for more than 95% of granulosa cell tumors and occurs more often in postmenopausal than premenopausal women, with a peak incidence between 50 and 55 years.1
Etiology
The etiology as well the molecular genetic events involved in the pathogenesis of granulosa cell tumor are unknown; however, amplification and/or over expression of the ERBB genes has been found in a number of granulosa cell tumors. For example, erbB4 was expressed in 10 of 12 tumors at moderate-to-high levels in more than 50% of cancer cells, whereas erbB2 and erbB3 were expressed less frequently in a single study.17 Several studies suggest that infertile women and those exposed to ovulation induction agents have an increased risk for granulosa cell tumor.1
Clinical features
Granulosa cell tumor may present as an abdominal mass with symptoms suggestive of functioning ovarian tumor. In approximately 5-15% of patients, hemoperitoneum may develop secondary to rupture of a cystic lesion.18Ascites occurs in about 10% of cases, and the tumor is asymptomatic in another 10%. Granulosa cell tumors are the most common estrogenic ovarian tumors clinically.
The symptoms and clinical manifestation vary according to the age of the patient and reproductive state. In prepubertal girls, granulosa cell tumor often induces isosexual pseudoprecocious puberty. In women of reproductive age, the tumor may be associated with menstrual disorders due to hyperestrinism. In postmenopausal women, irregular uterine bleeding is the most common manifestation due to endometrial hyperplasia or, rarely well-differentiated adenocarcinoma.
Gross findings
Granulosa cell tumors (GCTs) vary from tiny lesions to huge masses filling the abdomen, with a mean diameter of about 13 cm. GCTs are bilateral in only 2%. The external surface may be smooth or bosselated. The cut surface is solid, partly solid and partly cystic, or rarely, entirely cystic. Cystic tumors may be unilocular or multilocular. Solid areas may be hard and rubbery or soft in consistency and tend to be yellowish or gray in color; the cystic spaces may contain proteinaceous fluid or old altered blood. Hemorrhage and necrosis are common.19
Microscopic findings
Granulosa cells are small, usually round to polygonal, but may be spindle-shaped with scanty amphophilic cytoplasm and having indistinct cell borders. Their round, oval or angular nuclei often show a deep longitudinal grooves and may exhibit a small, variably prominent nucleolus. The nuclear chromatin may be compact and dense or loose and vesicular. Occasionally, tumors feature cells with enlarged, bizarre nuclei with multinucleated forms; these cells merge imperceptibly with more typical granulosa cells and are of no prognostic significance. The luteinized granulosa cells, seen particularly in the diffuse, multicystic, and ”juvenile” types of granulosa cell tumors, have larger, more rounded and generally ungrooved nuclei of similar general appearance and abundant pale eosinophilic lipid-laden cytoplasm.20
Etiology
The etiology as well the molecular genetic events involved in the pathogenesis of granulosa cell tumor are unknown; however, amplification and/or over expression of the ERBB genes has been found in a number of granulosa cell tumors. For example, erbB4 was expressed in 10 of 12 tumors at moderate-to-high levels in more than 50% of cancer cells, whereas erbB2 and erbB3 were expressed less frequently in a single study.17 Several studies suggest that infertile women and those exposed to ovulation induction agents have an increased risk for granulosa cell tumor.1
Clinical features
Granulosa cell tumor may present as an abdominal mass with symptoms suggestive of functioning ovarian tumor. In approximately 5-15% of patients, hemoperitoneum may develop secondary to rupture of a cystic lesion.18Ascites occurs in about 10% of cases, and the tumor is asymptomatic in another 10%. Granulosa cell tumors are the most common estrogenic ovarian tumors clinically.
The symptoms and clinical manifestation vary according to the age of the patient and reproductive state. In prepubertal girls, granulosa cell tumor often induces isosexual pseudoprecocious puberty. In women of reproductive age, the tumor may be associated with menstrual disorders due to hyperestrinism. In postmenopausal women, irregular uterine bleeding is the most common manifestation due to endometrial hyperplasia or, rarely well-differentiated adenocarcinoma.
Gross findings
Granulosa cell tumors (GCTs) vary from tiny lesions to huge masses filling the abdomen, with a mean diameter of about 13 cm. GCTs are bilateral in only 2%. The external surface may be smooth or bosselated. The cut surface is solid, partly solid and partly cystic, or rarely, entirely cystic. Cystic tumors may be unilocular or multilocular. Solid areas may be hard and rubbery or soft in consistency and tend to be yellowish or gray in color; the cystic spaces may contain proteinaceous fluid or old altered blood. Hemorrhage and necrosis are common.19
Microscopic findings
Granulosa cells are small, usually round to polygonal, but may be spindle-shaped with scanty amphophilic cytoplasm and having indistinct cell borders. Their round, oval or angular nuclei often show a deep longitudinal grooves and may exhibit a small, variably prominent nucleolus. The nuclear chromatin may be compact and dense or loose and vesicular. Occasionally, tumors feature cells with enlarged, bizarre nuclei with multinucleated forms; these cells merge imperceptibly with more typical granulosa cells and are of no prognostic significance. The luteinized granulosa cells, seen particularly in the diffuse, multicystic, and ”juvenile” types of granulosa cell tumors, have larger, more rounded and generally ungrooved nuclei of similar general appearance and abundant pale eosinophilic lipid-laden cytoplasm.20
The tumor cells grow in a variety of patterns, including microfollicular, macrofollicular, trabecular, insular, tubular, diffuse, moiré silk, and gyriform. The microfollicular variant, the most easily recognized, is characterized by multiple small rounded spaces formed by cystic degeneration in small aggregates of granulosa cells, containing eosinophilic PAS-positive material (chondroitin 6-sulphate) and often fragments of nuclear debris or pyknotic nuclei. These spaces, known as Call–Exner bodies, are found in only 30–50% of tumors.21 The granulosa cell nuclei are oriented somewhat radially around these structures (see Media file 1-2).
Granulosa cell tumor, microfollicular pattern. Call-Exner bodies are formed by a discrete array of granulosa cells with angular grooved nuclei and central inspissated eosinophilic material (H&E, x400).
Granulosa cell tumor, microfollicular pattern shows strong staining with inhibin (anti-inhibin, x400).
The macrofollicular variant comprises cysts of differing sizes lined by multilayered well-differentiated granulosa cells (see Media file 3-4), often cuffed by a layer of thecalike cells and apparently also formed by cystic degeneration in large masses of granulosa cells. Either or both of these cell layers may be luteinized.
Higher magnification of tumor depicted in Media file 2 shows a follicle lined by granulosa cells (H&E, x200).
Insular and trabecular variants exhibit islands and bands of granulosa cells with peripherally palisaded nuclei separated by a fibroma-like or thecoma-like stroma. Call–Exner bodies are common in this variant (see Media file 5). The watered-silk (moiré silk) and gyriform patterns (see Media file 6) are characterized by undulating parallel rows and zigzag cords of granulosa cells, respectively. The diffuse pattern is characterized by a monotonous sheetlike cellular proliferation (see Media file 7).
The cytologic features of GCT by fine needle aspiration cytology include naked nuclei, Call-Exner bodies, spindle-shaped hyperchromatic stromal cells within cellular clusters, blood vessels with prominent perivascular tumor cell growth, moderate-to-scant delicate cytoplasm, and small punctuate cytoplasmic vacuoles. Nuclei are generally centrally located, monotonous in size, and grooved with prominent central nucleoli.22
Immunohistochemistry
Granulosa cell tumors are positive for inhibin and calretinin but negative for cytokeratin 7 and epithelial membrane antigen. Keratin expression may be present but is usually focal, punctate, or patchy in distribution. Rare granulosa cell tumors may exhibit focal or patchy EMA expression, but diffuse positivity is highly unusual. The presence of smooth muscle actin and the absence of epithelial membrane antigen immunoreactivity are additional features that are characteristic of a granulosa cell tumor (GCT). S-100 protein immunoreactivity is a finding generally limited to GCTs among sex cord stromal tumors, and its presence may have some role in differentiating between Sertoli-stromal cell tumors and GCTs.
Because epithelial membrane antigen immunoreactivity is present in many of the histologic mimics of GCTs, such as metastatic or primary carcinoma, the absence of staining in GCT has diagnostic value. CD56 may be useful in distinguishing between granulosa cell tumor and normal ovarian follicles or endometrioid adenocarcinoma.23
Molecular/genetics
In contrast to older studies, recent karyotypic and fluorescence in situ hybridization analysis have shown that trisomy 12 is rarely present in granulosa cell tumor. Few studies have shown trisomy 14, monosomy 22, and structural changes in chromosome 6 with loss of 6q material.24,1
Because epithelial membrane antigen immunoreactivity is present in many of the histologic mimics of GCTs, such as metastatic or primary carcinoma, the absence of staining in GCT has diagnostic value. CD56 may be useful in distinguishing between granulosa cell tumor and normal ovarian follicles or endometrioid adenocarcinoma.23
Molecular/genetics
In contrast to older studies, recent karyotypic and fluorescence in situ hybridization analysis have shown that trisomy 12 is rarely present in granulosa cell tumor. Few studies have shown trisomy 14, monosomy 22, and structural changes in chromosome 6 with loss of 6q material.24,1
A somatic mutation in the FOXL2 gene has been identified in a large proportion of adult granulose cell tumors studied.25 A link has also been proposed between misregulated Wnt/beta-catenin signaling and GCT development.26
Coexistence of chromosome instability, microsatellite instability, and hypermethylation suggests that both genetic and epigenetic mechanisms may act in concert to inactivate the above-mentioned genes in these GCTs. These mechanisms can be an early event in the pathogenesis of these tumors, and it can be a critical step in the tumorigenic process.27
Prognosis and predictive factors
Granulosa cell tumors of all patterns have a malignant potential, with the capacity to extend beyond the ovary or recur after surgical removal; recurrent disease sometimes presents within 5 years of removal of the primary tumor, but more commonly it is not evident until much later, an average of 8-9 years. Distant metastases are rare but have been reported in the lungs (see Media file 8-10 ), brain, bones, and liver. Irrespective of the particular microscopic pattern observed, all granulosa cell tumors are considered as low-grade malignancies.
Higher magnification of the metastatic granulosa cell tumor shows nodules of granulosa cells with nuclear grooves (arrow) and brisk mitotic activity (H&Ex 400).
Metastatic ovarian granulosa cell tumor to the lung exhibits strong positive staining for inhibin while the rest of the lung is negative (anti-inhibin, x200).
The most important prognostic factor is the stage of the tumor; nearly 90% of patients with GCT have stage I disease. Factors related to a relatively poor prognosis include age over 40 years at the time of diagnosis, large tumor size (>5 cm), bilaterality, mitotic activity, and atypia.16,28
A recent study showed that no significant correlation existed between any of the proliferation indices (mitotic counts and Ki-67 immunoreactivity) and the clinical outcomes.29
A recent study showed that no significant correlation existed between any of the proliferation indices (mitotic counts and Ki-67 immunoreactivity) and the clinical outcomes.29
Serum inhibin levels are used to monitor patients for tumor recurrence.
Differentials
Diffuse adult granulosa cell tumors are often confused with undifferentiated carcinoma. The latter tumor is characterized by the presence of nuclear hyperchromatism, pleomorphism, and atypical mitotic figures in contrast with the typically pale, often grooved nuclei, absence of atypical mitotic activity, and the common association with endocrine manifestations of the granulosa cell tumor. Patients with undifferentiated carcinoma usually presented in advanced stage, while 90% of patients with granulosa cell tumor are stage I.20 In difficult cases, staining of undifferentiated carcinomas for EMA and lack of staining for α-inhibin in contrast to the results in granulosa cell tumors are helpful in the differential diagnosis.
The distinction between an endometrioid carcinoma with a microglandular pattern and microfollicular granulosa cell tumor is based on the presence of other characteristic patterns of either tumor, on the different nuclear features of the two tumors, and on staining pattern for inhibin. The nuclei of endometrioid carcinoma cells are round, hyperchromatic, and have more mitotic figures, while those of granulosa cell tumors are characteristically round, oval, or angular, pale, and often grooved (see Media file 11-12).
The distinction between an endometrioid carcinoma with a microglandular pattern and microfollicular granulosa cell tumor is based on the presence of other characteristic patterns of either tumor, on the different nuclear features of the two tumors, and on staining pattern for inhibin. The nuclei of endometrioid carcinoma cells are round, hyperchromatic, and have more mitotic figures, while those of granulosa cell tumors are characteristically round, oval, or angular, pale, and often grooved (see Media file 11-12).
Endometrioid carcinoma simulating an adult granulosa cell tumor with solid cellular areas punctuated by small spaces containing eosinophilic secretion similar to Call-Exner bodies (H&E, x200).
Endometrioid adenocarcinoma resembling granulosa cell tumor shows negative staining for inhibin (anti-inhibin, x400).
Cellular thecoma and fibroma may be difficult to distinguish from diffuse granulosa cell tumor; use of reticulin stains show abundant intercellular fibrils in the former tumors and scant reticulum in GCT.
Endometrioid stromal sarcomas may be misinterpreted as diffuse adult granulosa cell tumor. The lack of endocrine manifestations, the presence of many small arteries throughout the tumor (see Media file 13), and lack of staining for α-inhibin, and positive staining for CD10 are helpful in identifying the tumor as a stromal sarcoma.
Endometrioid stromal sarcoma shows diffuse proliferation of round-to-oval cells, which could be mistaken for cellular fibroma (H&E, x200).
The acini of carcinoid tumors may be mistaken for the Call-Exner bodies of adult granulosa cell tumors. The acini of carcinoid tumors often contain dense eosinophilic secretion, which is sometimes calcified; however, calcification is not a feature of the adult granulosa cell tumor. The nuclei of carcinoid tumors have stippled chromatin (salt and pepper appearance) in contrast to the pale grooved nuclei of adult granulosa cell tumors, and the cytoplasm is positive for neuroendocrine markers, unlike the cytoplasm of adult granulosa cell tumors, which is positive for inhibin.
Prominent microfollicular pattern in struma ovarii may result in confusion with the Call-Exner bodies of granulosa cell tumor. Features suggestive of struma ovarii include association with a dermoid cyst, demonstration of foci of typical thyroid follicles, lack of other characteristic patterns of the adult granulosa cell tumor, immunoreactivity for thyroglobulin and thyroid transcription factor-1 (TTF-1; see Media file 14-15), and negative staining for inhibin.30,13
Struma ovarii shows follicular and microacinar growth pattern. In some areas, the microfollicles contain dense, colloidlike material (H&E, x200).
Nests of transitional epithelial cells in benign Brenner tumors may mimic insular variant of GCT, in addition, the nuclei of transitional cells usually have longitudinal grooves; however, the nests in Brenner tumors are sharply demarcated and lie within an abundant fibrous stroma. The transitional cells have well-defined cell borders and eosinophilic-to-clear cytoplasm (see Media file 16). The epithelial nests stain typically for CK7 and are negative for inhibin.
Benign Brenner tumor shows sharply demarcated nests of transitional epithelial cells lying within an abundant fibrous stroma (H&E, x100).
Metastatic malignant melanoma and metastatic breast carcinoma, particularly those of the lobular type, may be confused with adult granulosa cell tumor as both tumors may contain cells with scanty cytoplasm that grow diffusely, mimicking the diffuse variant of adult granulosa cell tumor. The nuclei of malignant melanoma usually show nucleoli and the characteristic cytoplasmic pseudoinclusions; intracytoplasmic melanin pigment are usually found (see Media file 17-19); staining for α-inhibin and HMB-45 is helpful in problematic cases; however, adult granulosa cell tumors may stain for S-100 protein. Metastatic breast carcinomas usually stain positively for intracellular mucin, epithelial membrane antigen, and gross cystic disease fluid protein-15 and demonstrate negative staining for α-inhibin.31
A case of metastatic melanoma to the ovary shows ovary to the right infiltrated by nodule of malignant melanoma to the left with focal melanin pigment (H&E, x100).
Higher magnification of the metastatic melanoma shows eosinophilic cells with pleomorphic nuclei. Occasional nucleoli and cytoplasmic pseudoinclusions are present (H&E, x400).
Metastatic breast carcinoma shows diffuse infiltration by lobular-type epithelial cells, showing uniform nuclei with small but distinct nucleoli and eosinophilic cytoplasm (H&E x200).
Juvenile Granulosa Cell Tumor
EpidemiologyJuvenile granulosa cell tumor (JGCT) accounts for approximately 5% of all granulosa cell tumors. The neoplasm occurs mainly during the first 3 decades of life.1
Etiology
The etiology of JGCT is unknown. However, 2 modifier genes have been mapped in the chromosome X of mice that support juvenile-type GC tumor development in female mice.32
Clinical features
About 80% of JGCT occur in children, who typically present with isosexual pseudoprecocity. When JGCT occurs after puberty, patients usually present with abdominal pain, swelling, menstrual irregularities, or amenorrhea.
Gross findings
The appearance of juvenile granulosa cell tumor is not distinctive and is similar in its spectrum of appearances to the adult variant.
Microscopic findings
JGCT is typically a solid cellular neoplasm, with focal follicle formation. The follicles are of variable sizes and shapes but generally don’t reach the large size of the follicles in the macrofollicular variant of adult granulosa cell tumor. Their lumens contain basophilic or eosinophilic fluid. Variable layers of granulosa cells line the follicles and occasionally surrounded by mantle of theca cells. The neoplastic cells in the solid areas may be arranged diffusely or divided into nodules by fibrous septa. A fibrothecomatous stroma with variable luteinization and/or edema is often evident.
In some tumors, luteinization is so extensive that follicles may be few and poorly developed. The neoplastic granulosa cells have abundant eosinophilic and/or vacuolated cytoplasm and rounded hyperchromatic nuclei. Nuclear grooves are rare. Nuclear atypia in JGCTs varies from minimal to marked. The mitotic rate is also variable but is generally higher than adult granulosa cell tumor (AGCT; see Media file 20-23).
In some tumors, luteinization is so extensive that follicles may be few and poorly developed. The neoplastic granulosa cells have abundant eosinophilic and/or vacuolated cytoplasm and rounded hyperchromatic nuclei. Nuclear grooves are rare. Nuclear atypia in JGCTs varies from minimal to marked. The mitotic rate is also variable but is generally higher than adult granulosa cell tumor (AGCT; see Media file 20-23).
Juvenile granulosa cell tumor shows round-to-oval follicles that contain eosinophilic secretion; the follicles are separated by luteinized granulosa cells (H&E, x200).
Juvenile granulosa cell tumor. Follicles of varying sizes and shapes are separated by cellular areas (H&E, x40).
Juvenile granulosa cell tumor. The cells have abundant eosinophilic cytoplasm; their nuclei are hyperchromatic and lack grooves (H&E, x400).
Immunohistochemistry
JGCTs often exhibit strong and diffuse CD99 positivity. EMA expression is also more common in JGCT than in AGCT.
Molecular and genetics
An association between tuberous sclerosis and juvenile granulosa cell tumor of the ovary has been reported.33JGCT may present as a component of a variety of nonhereditary congenital syndromes, including Ollier disease (enchondromatosis) and Maffucci syndrome (enchondromatosis and hemangiomatosis). Bilateral JGCT may develop in infants with features suggestive of Goldenhar (craniofacial and skeletal abnormalities) or Potter syndrome.1
Prognosis and predictive factors
Most JGCT are clinically benign. As in AGCT, the most important prognostic factor is the stage of the tumor.
In contrast to AGCT, which recur late, almost all clinically malignant JGCT recur within 3 years.
Differentials
JGCT may be confused with AGCT. The follicles of JGCT are more irregular in size and shape than those of an adult granulosa cell tumor; a myxoid background and luteinization is also more common in JGCT. JGCT nuclei are round and more hyperchromatic, and lack nuclear grooves. Call-Exner bodies are absent in JGCT.31 Differentials
Thecoma is distinguished from a solid JGCT on the basis of the distribution of reticulin, as seen on reticulin stain: reticulin fibers typically surround individual cells in fibromas and thecomas, but in granulosa cell tumors, the reticulin fibers surround nests of cells.
JGCT may be misdiagnosed as a malignant germ cell tumor, usually a yolk sac tumor or, less commonly, embryonal carcinoma. The nuclei of the JGCT are usually not as primitive as those of either germ cell tumor. The presence of typical patterns of embryonal carcinoma (solid, papillary, and glandular with necrosis) and yolk sac tumor (reticular, glandular, Schiller-Duval bodies) help establish these diagnoses; in more difficult cases, the immunohistochemical demonstration of CD30 in embryonal carcinoma; alpha- fetoprotein and glypican-3 in yolk sac tumor; and SALL4 in embryonal carcinoma and yolk sac tumor are useful.34 Yolk sac tumor and embryonal carcinoma do not express inhibin.20,35
JGCT may be misinterpreted as one of the surface epithelial tumors, especially clear cell carcinoma, and, rarely, transitional cell carcinoma or undifferentiated carcinoma; however, these carcinomas are rare in the age group in which JGCTs occur and have morphological and immunohistochemical profiles that differ from those of JGCT.
Small cell carcinoma of hypercalcemic type may mimic JGCT because of the presence of folliclelike spaces and predilection for young patients. However, small cell carcinoma of hypercalcemic type is often associated with hypercalcemia, while JGCT is usually associated with estrogenic manifestations. In addition, small cell carcinoma is composed of cells with scanty cytoplasm distributed in sheets, while JGCT features cells with moderate-to-large amounts of cytoplasm arranged in nodules and large, irregular follicles (see Media file 24). Small cell carcinoma, hypercalcemic type, does not express inhibin.
Small cell carcinoma, hypercalcemic type shows diffuse proliferation of small cells with scant cytoplasm and hyperchromatic nuclei with single, small nucleoli and focal follicle formation (H&E, x400).
Primary and metastatic melanoma can simulate JGCT; bilateral ovarian involvement, prior history of melanoma, melanin pigment, and prominent expression of melanocytic markers usually provide sufficient support for metastatic melanoma. Use of several differential markers is suggested, since JGCT may express S100 protein and melanoma may express inhibin.
Thecoma-Fibroma Group
Thecoma
Definition
Thecoma is a benign tumor composed of plump spindle cells with obvious lipid-containing cytoplasm and resembling cells of the ovarian theca interna.20
Thecoma is a benign tumor composed of plump spindle cells with obvious lipid-containing cytoplasm and resembling cells of the ovarian theca interna.20
Epidemiology
Thecoma occurs at any age but predominantly over the age of 40 years and rarely in children. Luteinized variants are more likely to occur in younger women. Estrogenic manifestations are the commonest mode of clinical presentation and usually take the form of menstrual irregularities or postmenopausal bleeding. Androgenic thecomas are rare.36
Clinical featuresThecoma occurs at any age but predominantly over the age of 40 years and rarely in children. Luteinized variants are more likely to occur in younger women. Estrogenic manifestations are the commonest mode of clinical presentation and usually take the form of menstrual irregularities or postmenopausal bleeding. Androgenic thecomas are rare.36
Estrogenic manifestations are the commonest mode of clinical presentation for thecoma and usually take the form of menstrual irregularities or postmenopausal bleeding. Androgenic thecomas are rare.
Gross findings
Thecomas range in size from small nodules to large, solid, firm or rubbery tumors several centimeters in diameter. The cut surface is characteristically bright yellow to orange. They are usually unilateral.
Microscopic findings
Thecoma has 1 of 2 patterns. Typical thecomas consist of large, ill-defined, nodular masses of plump eosinophilic or vacuolated cells with small, pale, round, or ovoid, central, nongrooved nuclei, interspersed between less conspicuous bands of fibrous connective tissue, which often exhibit hyalinized plaques (see Media file 25). Reticulin stains show a fine meshwork of fibrils investing individual cells, in contrast to granulosa cell tumor. Mitoses tend to be rare. Edema or myxoid change is often prominent and some tumors show an occasional focus of dystrophic calcification. Occasional small nests of granulosa cells may be found; such tumors are called “thecomas with minor sex cord elements.”37
The second defined histological pattern, designated luteinized thecoma, is a typical thecoma throughout which clusters of large eosinophilic, lipid-laden lutein cells are scattered. These tumors are unassociated with a background of stromal hyperplasia and therefore distinguishable from stromal luteomas.
A rare subtype of luteinized thecoma is associated with sclerosing peritonitis, this type is usually bilateral, in contrast to the thecoma and luteinized thecoma of the usual type. Microscopic examination of this lesion reveals alternating hypercellular and hypocellular areas, with edema and microcystic changes. The mitotic count is usually elevated; however, no evidence exists that this lesion has the ability to metastasize.38,39,40
Prognosis and predictive factors
Thecomas are benign. Luteinizing thecoma with sclerosing peritonitis requires more extensive surgery due to the associated adhesions and obstructive symptoms. Rare patients have died of complications of the peritoneal sclerosis.
Differentials
The chief differential diagnosis is adult granulosa cell tumor with a diffuse pattern. Nuclear grooves are characteristic of adult granulosa cell tumor, but not thecoma. However, the most useful distinguishing feature is the pattern of reticulin fibers on reticulin stain: in thecomas reticulin invests individual cells, while in granulosa cell tumors, the reticulin surrounds aggregates of cells.
Sclerosing stromal tumor exhibits a more prominent lobular pattern and occurs at a younger age than most thecomas. Sclerosing stromal tumor also exhibits a more prominent vascular pattern than thecoma.
Stromal hyperthecosis is distinguished from luteinized thecoma with sclerosing peritonitis on the basis of bilaterality, absence of ascites or peritoneal sclerosis, and absence of mitotic figures.
Fibroma
EpidemiologyFibromas are the most commonly encountered subtype of the sex cord-stromal tumors, accounting for almost two-thirds of neoplasms in this group. The mean age of incidence is about 48 years. Ascites is the most frequent general abdominal symptom associated with ovarian fibromas, being present in over 10% of cases.41
Hereditary basal cell nevus syndrome is statistically associated with an increased risk of ovarian fibroma.42,43
Gross findings
Fibromas are bilateral in 5% of cases and average 6 cm in diameter. Larger tumors have a smooth or slightly bosselated serosal surface, are solid, and vary from edematous and somewhat rubbery to rock-hard in consistency. The cut surface is white and faintly whorled, often with areas of cystic degeneration.
Fibromas are bilateral in 5% of cases and average 6 cm in diameter. Larger tumors have a smooth or slightly bosselated serosal surface, are solid, and vary from edematous and somewhat rubbery to rock-hard in consistency. The cut surface is white and faintly whorled, often with areas of cystic degeneration.
Microscopic findings
The basic architectural pattern of fibroma comprises variably cellular bundles and intersecting collagenous fibrous tissue, occasionally with a striking storiform pattern resembling ovarian cortex. The elongated fibroblastic tumor cells have spindle-shaped nuclei and may contain small amounts of lipid in their cytoplasm (see Media file 26). Dystrophic calcification may be occasionally seen. Nuclear atypia and mitoses are rarely seen.44
Fibroma. The cells have small, spindle-shaped nuclei lacking atypia or mitotic activity (H&E, x100).
Occasional fibromatous tumors are hypercellular and/or show appreciable mitotic activity. These tumors are designated as cellular fibromas, mitotically active cellular fibromas, or fibrosarcomas, depending on the degree of mitotic activity and nuclear atypia. Cellular fibromas contain 1–3 mitotic figures per 10 high-power fields but display bland nuclei (see Media file 27). Mitotically active cellular fibromas contain 4-19 mitotic figures per 10 high-power fields. Both variants may exhibit foci of necrosis, but nuclear atypia is minimal or absent.
Fibrosarcomas have increased cellularity, often a high mitotic rate, and diffuse nuclear hyperchromasia with atypia. The latter tumors tend to be large, solid tumor masses accompanied by dense adhesions.45
Molecular and genetics
Trisomy 12 is a common finding in benign and cellular fibromas, while trisomy 8 has been identified in cases of clinically malignant fibrosarcomas.46,47
Prognosis
Fibromas are benign. Cellular and cellular mitotically active fibromas are also clinically benign, although tumor rupture and/or adhesions may be associated with local recurrence. Fibrosarcoma often has a malignant clinical course.
Differential Diagnosis
Fibroma should be distinguished from stromal hyperplasia. Stromal hyperplasia is bilateral, involves cortex and medulla, and is often an incidental finding in ovaries removed from postmenopausal women undergoing surgery for other reasons. Nodularity may be present, but the collagenized stroma and hyaline plaques seen in fibroma are absent.Fibroma and thecoma may show histologic overlap; the term “fibrothecoma” is often used for such tumors. Presence of a nodular growth pattern, abundant pale or eosinophilic cytoplasm, and strong inhibin positivity are more typical for thecoma than fibroma.
Massive ovarian edema with fibromatosis is distinguished from fibroma by the preservation of normal ovarian structures; the former lesion surrounds and envelopes ovarian follicles, while fibroma displaces (or effaces) them.
Stromal Tumors With Minor Sex Cord Elements
Some stromal tumors contain minor sex cord elements.48 By definition, the sex cord element should account for less than 10% of the tumor on any one slide examined. Most such tumors resemble fibromas, but occasional tumors are thecomatous. Estrogenic manifestations may be present. The prognosis of stromal tumors with minor sex cord elements should be that of the underlying stromal tumor.48 None of the patients reported to date have had any evidence of tumor recurrence; however, follow-up was limited.49
Microscopic findings
Microscopic findings
The sex cord elements may be composed of fully differentiated granulosa cells with nuclear grooves, have a totally indifferent appearance, or form solid tubular structures resembling immature testicular tubules. In some instances, the fibromalike component may contain clusters of lutein cells. Rarely, a thecoma can contain minor sex cord elements.
Sclerosing Stromal Tumor
DefinitionSclerosing stromal tumor (SST) of the ovary is a very rare sex cord stromal tumor occurring in a younger age group than other types of stromal tumors and most commonly accompanied by menstrual irregularity and characterized by several unique histologic features including pseudolobulation, sclerosis, and prominent vascularity.
Gross findings
Sclerosing stromal tumor is usually unilateral, rarely bilateral, and well-circumscribed.50 The cut section is solid and white with areas of edema, cyst formation, and yellowish discoloration.
Microscopic findings
Sclerosing stromal tumor is usually unilateral, rarely bilateral, and well-circumscribed.50 The cut section is solid and white with areas of edema, cyst formation, and yellowish discoloration.
Microscopic findings
Sclerosing stromal tumor is characterized by the presence of pseudolobular pattern in which cellular nodules were separated by areas of densely collagenous or edematous connective tissue, which contained fewer cells. The nodules are composed of an admixture of fibroblasts and rounded vacuolated cells, with prominent thin-walled blood vessels.51
Prognosis and predictive factorsSclerosing stromal tumor is benign.1
Differential diagnosis
Luteinized thecoma may resemble sclerosing stromal tumor, but the latter exhibits a more variegated appearance with prominent vascular pattern.Fibroma occurs in older women and exhibits more diffuse fibrosis with hyalinized plaques. A prominent vascular pattern is not seen.
Metastatic signet ring carcinoma may mimic a cellular fibroma or fibrothecoma. Diagnostic features of metastatic carcinoma include bilateral ovarian involvement, presence of signet ring cells, or small glandular structures on careful microscopic examination, and cytokeratin expression on immunohistochemical stains.
Signet-Ring Stromal Tumor
DefinitionSignet-ring stromal tumor was the term proposed by Ramzy (1976) to designate an unusual benign ovarian neoplasm of probable stromal origin, characterized by the presence of signet-ring cells that did not stain for lipid or mucin.
Gross findings
Signet-ring stromal tumor may be solid or solid and cystic.
Microscopic findings
Signet-ring stromal tumor may be solid or solid and cystic.
Microscopic findings
Signet-ring stromal tumor is composed of an admixture of spindle and round cells. Some of the round cells show a typical signet-ring appearance. The spindle cells have elongated nuclei, nonvacuolated cytoplasm, and are arranged in fascicles. The round cells usually have a single clear vacuole, which compresses the nucleus and/or single or multiple intracytoplasmic eosinophilic globules. Spindle and round cells are surrounded by reticulin fibers. The vacuoles are negative for mucin stains, a feature which distinguishes this tumor from Krukenberg tumor.52
Sertoli-Stromal Cell Tumors
Definition
Sertoli-stromal cell tumors are tumors containing in pure form or in various combinations Sertoli cells, cells resembling rete epithelial cells, cells resembling fibroblasts, and Leydig cells in variable degree of differentiation.1
Sertoli Cell Tumors
Sertoli cell tumors typically occur in young patients with a mean of 30 years; however, a wide age range exists. The tumors are more commonly estrogenic than androgenic.53Gross findings
Sertoli cell tumors vary markedly in size, but most are smaller than 10 cm in diameter. They tend to be solid, firm, encapsulated and lobulated masses, typically yellow or tan in color. Small cystic areas are infrequently present. They are typically unilateral, but may be bilateral in phenotypic females with testicular feminization.
Microscopic findingsSertoli cell tumors are composed of lobules of uniform, solid, or hollow tubules, lined by one or more layers of cuboidal to columnar benign-appearing cells with eosinophilic or vacuolated cytoplasm and dark, oval, basal nuclei. Mitoses are rare and nucleoli are not prominent (see Media file 28-29). Sertoli cells usually contain lipid droplets but, in some tumors, they are greatly distended by fat, giving rise to the so-called Sertoli cell tumor with lipid storage or lipid-rich Sertoli cell tumor. Intervening stroma is usually represented by a few bands of acellular connective tissue that is either edematous or hyalinized and contains few or no Leydig cells. If more than rare Leydig cells are present, the diagnosis of well-differentiated Sertoli-Leydig cell tumor should be made. The oxyphilic variant of Sertoli cell tumors have been described in women with Peutz-Jeghers syndrome.54
Sertoli cell tumor shows closely packed hollow and solid tubules lined by well-differentiated cuboidal-to-columnar epithelial cells. Leydig cell are absent (H&E, x400).
Well-differentiated Sertoli cell tumor with arrow that shows strong cytoplasmic staining for inhibin in both Sertoli and Leydig cells (anti-inhibin, x200).
Prognosis and predictive factors
Well-differentiated Sertoli cell tumors are benign, whereas those exhibiting atypical features are frequently presented with advanced stage disease and exhibit aggressive behavior.55 The histologic features of ovarian Sertoli cell tumors that best correlate with adverse outcome are nuclear atypia, 5 or more mitotic figures per 10 high power fields, and tumor necrosis.53 Bizarre nuclei of degenerative type unassociated with an elevated mitotic index do not influence the prognosis.49
Differentials
The differential diagnosis for Sertoli cell tumors includes endometrioid carcinoma, well differentiated Sertoli-Leydig cell tumor, carcinoid tumor, and female adnexal tumor of probable wolffian origin.Endometrioid carcinoma typically occurs at an older age, may be associated with endometriosis, and can often be recognized by the presence of squamous or morular metaplasia. In contrast to Sertoli cell tumors, endometrioid carcinomas are positive for cytokeratin 7 and EMA and are negative for inhibin.
Sertoli-Leydig cell tumor exhibits a heterogenous pattern of Sertoli cell tubules and more than occasional Leydig cells; heterologous elements may also be present.
Carcinoid tumors (primary or metastatic) exhibit typical coarse and fine chromatin patterns, often in a fibromatous stroma. Primary carcinoid tumors are often associated with a dermoid cyst. Confirmation of neuroendocrine differentiation can be accomplished with synaptophysin and chromogranin stains for neurosecretory granules.
Female adnexal tumor of probable wolffian origin arises primarily in the broad ligament but may present as an ovarian mass. The architecture varies from cystic, to a slitlike glandular or a sievelike pattern. The constituent cells are small, oval, or spindle shaped. Inhibin expression may be present but is usually weak.
Sertoli–Leydig Cell Tumor Group
EpidemiologySertoli-Leydig cell tumors are uncommon benign tumors, accounting for less than 0.5% of all ovarian tumors. The reported mean age is 23-25 years in different studies.56 The well-differentiated tumors occur at an average age of 35 years and retiform tumors at an average age of 15 years.31
Clinical features
About one third of patients with Sertoli-Leydig cell tumor have androgenic manifestations including: oligomenorrhea that may proceed into amenorrhea, hirsutism, hoarseness, breast atrophy, and clitoral hypertrophy. Approximately half of patients with Sertoli-Leydig cell tumor have no endocrine manifestation and mainly experience abdominal swelling and pain. Occasional patients have been represented with estrogenic manifestation, including irregular menstruation or menorrhagia in women in the reproductive age group and postmenopausal bleeding in older women.
Well-Differentiated Sertoli–Leydig Cell Tumors
Well-differentiated Sertoli–Leydig cell tumors account for about 10% of all Sertoli–Leydig cell tumors. There is a statistical association with congenital anomaly of the internal genitalia in otherwise normal women and with the testicular feminization syndrome.57
Gross findings
These tumors are usually unilateral, solid, well-circumscribed, yellow, firm, and lobulated masses of 5–6 cm average size. The cut surface shows hemorrhage and cystification.58
Microscopic findings These tumors are usually unilateral, solid, well-circumscribed, yellow, firm, and lobulated masses of 5–6 cm average size. The cut surface shows hemorrhage and cystification.58
Well-differentiated Sertoli–Leydig cell tumor consists of uniform solid or hollow tubular structures lined by Sertoli-type cells. The intervening stroma contains variable numbers of Leydig cells. The latter tend to be packed in nests between the Sertoli cell tubules but may form more solid sheets in some tumors. Mitoses are rare in these tumors.57
Moderately and Poorly Differentiated Sertoli–Leydig Cell Tumors
Gross findings
These tumors are bilateral in less than 2% of cases, circumscribed, partly solid, and pa rtly cystic with a bosselated outer surface. The mean diameter is about 15 cm. Polypoid masses may project into the cystic spaces. Solid portions of the tumor are lobulated, firm or fleshy in consistency, and usually yellow-gray in color. Necrosis and hemorrhage are often prominent.
Microscopic findings
Moderately differentiated Sertoli–Leydig cell tumors usually exhibit cellular nodules or ”lobules” separated by zones of loose fibrous or fibromyxoid mesenchymal stroma. Immature Sertoli-type cells, with small oval or angular nuclei and either scanty or rather more obvious pale cytoplasm are arranged in short, thin cords resembling the sex cords of the immature testes and having double rows of cells with nuclei arranged antipodally. Nuclei are relatively bland and mitoses, which are infrequent, average about 5 mitotic figures per 10 high-power fields.
Mature Leydig cells are usually apparent in the stroma, particularly around the perimeter of the tumor or at the margins of cellular nodules, as sheets, clusters, or single cells, and less commonly, within the cellular zones (seeMedia file 30-31). The poorly differentiated tumors show spindle-shaped immature Sertoli cells that may exhibit nuclear atypia and mitotic activity admixed, with clusters of Leydig cells with abundant eosinophilic cytoplasm (see Media file 32-33).
These tumors are bilateral in less than 2% of cases, circumscribed, partly solid, and pa rtly cystic with a bosselated outer surface. The mean diameter is about 15 cm. Polypoid masses may project into the cystic spaces. Solid portions of the tumor are lobulated, firm or fleshy in consistency, and usually yellow-gray in color. Necrosis and hemorrhage are often prominent.
Microscopic findings
Moderately differentiated Sertoli–Leydig cell tumors usually exhibit cellular nodules or ”lobules” separated by zones of loose fibrous or fibromyxoid mesenchymal stroma. Immature Sertoli-type cells, with small oval or angular nuclei and either scanty or rather more obvious pale cytoplasm are arranged in short, thin cords resembling the sex cords of the immature testes and having double rows of cells with nuclei arranged antipodally. Nuclei are relatively bland and mitoses, which are infrequent, average about 5 mitotic figures per 10 high-power fields.
Mature Leydig cells are usually apparent in the stroma, particularly around the perimeter of the tumor or at the margins of cellular nodules, as sheets, clusters, or single cells, and less commonly, within the cellular zones (seeMedia file 30-31). The poorly differentiated tumors show spindle-shaped immature Sertoli cells that may exhibit nuclear atypia and mitotic activity admixed, with clusters of Leydig cells with abundant eosinophilic cytoplasm (see Media file 32-33).
Low magnification of Sertoli-Leydig cell tumor of intermediate differentiation shows cellular lobules separated by stroma (H&E, x40).
Higher magnification of Sertoli-Leydig cell tumor of intermediate differentiation shows clusters of immature Sertoli cells admixed with hollow and solid tubules; Leydig cells indicated by arrow are present in between the Sertoli cells (H&E, x200).
Poorly differentiated Sertoli-Leydig cell tumor shows diffuse solid proliferation of immature Sertoli cells (H&E, x100).
Higher magnification of the poorly differentiated Sertoli-Leydig cell tumor shows nests and cord of immature Sertoli cells. Occasional Leydig cells with eosinophilic cytoplasm are seen in between the Sertoli cells (H&E, x200).
Sertoli-Leydig Cell Tumor With Heterologous Elements
These tumors are separable from other moderately and poorly differentiated Sertoli–Leydig cell tumors on purely histological grounds, namely the presence of heterologous elements, the most common of which is gastrointestinal mucin-secreting epithelium. Mucinous heterologous elements are seen in about 20% of Sertoli–Leydig cell tumors. Approximately, 5% of cases contain immature skeletal muscle and/or cartilage. Rarely do they exhibit neuroblastoma, fat, carcinoid, smooth muscle, bone, hepatocytes, and endometrium or endometrioid adenofibroma.59
Gross findings
Part or the entire cystic component of a Sertoli-Leydig cell tumor may be mucinous in type; however, heterologous elements are only occasionally diagnosed macroscopically.1
Part or the entire cystic component of a Sertoli-Leydig cell tumor may be mucinous in type; however, heterologous elements are only occasionally diagnosed macroscopically.1
Microscopic findings
Heterologous mesenchymal elements usually consist of mucinous epithelium, cartilage, skeletal muscle or rhabdomyosarcoma. They may be admixed with the sex cord area of the tumor or present as discrete areas. The mucinous epithelium is usually bland, intestinal, or gastric-type epithelium, but sometimes shows borderline or malignant changes (see Media file 34-36). The wide range of tissue types seen in immature teratomas is characteristically absent, thus distinguishing these 2 entities. If the Sertoli–Leydig cell component is small and overlooked, the tumor may be mistyped as a pure sarcoma.20
Heterologous mesenchymal elements usually consist of mucinous epithelium, cartilage, skeletal muscle or rhabdomyosarcoma. They may be admixed with the sex cord area of the tumor or present as discrete areas. The mucinous epithelium is usually bland, intestinal, or gastric-type epithelium, but sometimes shows borderline or malignant changes (see Media file 34-36). The wide range of tissue types seen in immature teratomas is characteristically absent, thus distinguishing these 2 entities. If the Sertoli–Leydig cell component is small and overlooked, the tumor may be mistyped as a pure sarcoma.20
Moderately differentiated Sertoli-Leydig cell tumor with heterologous elements. Note the presence of the epithelial glands in the middle of the field (H&E, x5).
Higher magnification of moderately differentiated Sertoli-Leydig cell tumor with heterologous elements shows admixture of glands lined by well-differentiated mucinous epithelial cells with interspersed goblet cells, Sertoli cells, and Leydig cells, indicated by the arrow (H&E, x200).
Moderately differentiated SLCT with heterologous elements shows moderate staining of the Sertoli component, strong staining of the Leydig cells, and negative staining of the epithelial glands for inhibin (anti-inhibin, x200).
Retiform Variants
Fourteen percent of moderately and 30% of poorly differentiated ovarian Sertoli–Leydig cell tumors exhibit retiform foci, so-called because of a resemblance to the rete testis. Tumors with this pattern occur at a slightly younger age (mean age of 15 years) than those without and are less likely to produce clinical signs of virilization.59
Microscopically, this pattern is typified by an irregular network of slit-like spaces and cysts, often containing papillae of various shapes. The cystic and compressed tubular spaces and the papillae are lined by flattened or cuboidal cells with the same general cytological features as the immature Sertoli cells. Papillae may be short and rounded with hyalinized cores (see Media file 37), large and bulbous with edematous cores, or more complex and branched, occasionally simulating those of serous or endometrioid epithelial carcinomas.60,61
Retiform Sertoli-Leydig cell tumor shows admixtures of branching retiform tubules and papillary areas with prominent hyalinized cores (H&E, x200).
Prognosis and predictive factors
The incidence of clinical malignancy in Sertoli stromal cell tumors is 10–30%. The most reliable indication of malignancy is evidence of local extraovarian spread or metastases at the time of staging laparotomy. Histological grade correlates to some extent with the likely clinical outcome; 11% of moderately differentiated tumors are clinically malignant, while 20% of those with heterologous mesenchymal elements and 60% of poorly differentiated tumors are clinically malignant.62,1
Differentials
Sertoliform endometrioid carcinoma, first described by Roth in 1982 refers to a specific type of endometrioid carcinoma that has a pattern similar to that of sex cord-stromal tumors, particularly the Sertoli-Leydig cell tumor. These tumors contain extensive areas of compact, anastomosing slender cords with a stratified cell pattern that can be misinterpreted as a Sertoli-Leydig cell tumor. Typically, areas of well-differentiated, conventional endometrioid carcinoma are present with occasional foci of squamous metaplasia. Positive staining for cytokeratin 7 and negative staining for inhibin (see Media file 38-39) usually establish the correct diagnosis.63,64
Differentials
Sertoliform endometrioid carcinoma, first described by Roth in 1982 refers to a specific type of endometrioid carcinoma that has a pattern similar to that of sex cord-stromal tumors, particularly the Sertoli-Leydig cell tumor. These tumors contain extensive areas of compact, anastomosing slender cords with a stratified cell pattern that can be misinterpreted as a Sertoli-Leydig cell tumor. Typically, areas of well-differentiated, conventional endometrioid carcinoma are present with occasional foci of squamous metaplasia. Positive staining for cytokeratin 7 and negative staining for inhibin (see Media file 38-39) usually establish the correct diagnosis.63,64
Sertoliform endometrioid carcinoma demonstrating negative staining for inhibin; note positive staining of the stromal cells, indicated by the arrow (anti-inhibin, x400).
The tubular Krukenberg tumor may mimic a Sertoli-stromal cell tumor, particularly if intracellular mucin is not evident on routine staining. Tubular Krukenberg tumors are frequently bilateral, and compulsive sampling of the tumor usually demonstrates the typical signet-ring cells filled with mucin.
Retiform Sertoli-Leydig cell tumor may be misdiagnosed as yolk sac tumor or serous adenocarcinoma. The presence of more typical areas of Sertoli-Leydig cell tumor and positive staining for inhibin are diagnostic. In contrast, yolk sac tumors express AFP, glypigan-3, and SALL4, while most serous carcinomas express cytokeratin 7 and EMA.
Retiform Sertoli-Leydig cell tumor may be misdiagnosed as yolk sac tumor or serous adenocarcinoma. The presence of more typical areas of Sertoli-Leydig cell tumor and positive staining for inhibin are diagnostic. In contrast, yolk sac tumors express AFP, glypigan-3, and SALL4, while most serous carcinomas express cytokeratin 7 and EMA.
Sertoli-Leydig cell tumors with heterologous elements are often mistaken for teratomas; in contrast to Sertoli-Leydig cell tumors, teratomas also contain foci of squamous, respiratory, and skin appendageal differentiation. Poorly-differentiated Sertoli- Leydig cell tumors with heterologous elements may be misdiagnosed as carcinosarcoma or malignant mesodermal mixed tumor (MMMT). However, the former are commonly virilizing; moreover, carcinosarcoma usually occurs in older patients than those with Sertoli- Leydig cell tumor.
Carcinoid tumors of the trabecular type may be misdiagnosed as Sertoli- Leydig cell tumors of intermediate differentiation. The ribbons of the former, however, are much longer and more uniformly distributed than sex cord-like formations and the stroma does not contain Leydig cells. Positive staining for chromogranin and synaptophysin and negative staining for inhibin are additional corroborative features.31
Ovarian tumors of probable wolffian origin may be mistaken for Sertoli- stromal cell tumors because of presence of solid and hollow tubules in the former; however, the wolffian tumors lack Leydig cells, are rarely associated with endocrine manifestations, and additional sampling of the tumor usually demonstrates the distinctive sievelike pattern of a tumor of probable wolffian origin (see Media file 40).
Ovarian tumor of probable wolffian origin composed of uniform epithelial cells arranged in irregular tubules, nests, and cords (H&E, x100).
Sex Cord-Stromal Tumors of Mixed or Unclassified Cell Types
Definition
This group of sex cord-stromal tumors do not fall in the granulosa-stromal, Sertoli-stromal, or steroid cell category.1
This group of sex cord-stromal tumors do not fall in the granulosa-stromal, Sertoli-stromal, or steroid cell category.1
Sex Cord Tumor With Annular Tubules
Definition
Sex cord tumor with annular tubules (SCTAT) is a tumor composed of sex cord (Sertoli) cells arranged in simple and complex annular tubules.65
Sex cord tumor with annular tubules (SCTAT) is a tumor composed of sex cord (Sertoli) cells arranged in simple and complex annular tubules.65
Epidemiology
Sex cord tumors with annular tubules occur in 2 clinical settings. Firstly, they occur in almost all female patients with the Peutz–Jeghers syndrome (generalized hamartomatous intestinal polyposis and melanin spots of the oral mucosa, lips and digits), such cases account for about one-third of reported examples of ovarian SCTAT. The tumors may occur at almost any age, but the mean age at presentation is 27 years.1 Secondly, cases not associated with the Peutz–Jeghers syndrome (PJS) occur at mean age of 34 years. Over one-half of patients give a history of postmenopausal bleeding, menstrual irregularities or isosexual pseudopuberty, suggesting hyperestrogenism.66,67
Gross findings
Sex cord tumors with annular tubules, which occur in conjunction with the Peutz–Jeghers syndrome, are usually multifocal, bilateral and almost always very small tumorlets found incidentally in ovaries. In patients without the Peutz–Jeghers syndrome, SCTAT is usually unilateral and presents as a solitary, large solid mass up to 33 cm in diameter. The cut section of the tumor is solid and yellow.
Microscopic picture
SCTAT typically exhibits well circumscribed, rounded or oval, epithelial islands made up of ring-shaped, lumenless tubules encircling glassy, acidophilic, PAS-positive, basement membrane-like material. The rings themselves are set in fibrous ovarian stroma. This stroma may contain foci of luteinized cells and show focal hyalinization.68Sometimes, simple annular cords envelope single deposits of hyaline material, but, more commonly, a network of complex tubules interdigitate with many such deposits. The cytoplasm is abundant, pale, and vacuolated or slightly granular. Regular, rounded, occasionally grooved nuclei, often with a single small nucleolus, are generally arranged in a double row: one row at the periphery of the cell nests and the second around the hyaline deposits. Mitoses are rare (see Media file 41-42).
Sex cord tumors with annular tubules occur in 2 clinical settings. Firstly, they occur in almost all female patients with the Peutz–Jeghers syndrome (generalized hamartomatous intestinal polyposis and melanin spots of the oral mucosa, lips and digits), such cases account for about one-third of reported examples of ovarian SCTAT. The tumors may occur at almost any age, but the mean age at presentation is 27 years.1 Secondly, cases not associated with the Peutz–Jeghers syndrome (PJS) occur at mean age of 34 years. Over one-half of patients give a history of postmenopausal bleeding, menstrual irregularities or isosexual pseudopuberty, suggesting hyperestrogenism.66,67
Gross findings
Sex cord tumors with annular tubules, which occur in conjunction with the Peutz–Jeghers syndrome, are usually multifocal, bilateral and almost always very small tumorlets found incidentally in ovaries. In patients without the Peutz–Jeghers syndrome, SCTAT is usually unilateral and presents as a solitary, large solid mass up to 33 cm in diameter. The cut section of the tumor is solid and yellow.
Microscopic picture
SCTAT typically exhibits well circumscribed, rounded or oval, epithelial islands made up of ring-shaped, lumenless tubules encircling glassy, acidophilic, PAS-positive, basement membrane-like material. The rings themselves are set in fibrous ovarian stroma. This stroma may contain foci of luteinized cells and show focal hyalinization.68Sometimes, simple annular cords envelope single deposits of hyaline material, but, more commonly, a network of complex tubules interdigitate with many such deposits. The cytoplasm is abundant, pale, and vacuolated or slightly granular. Regular, rounded, occasionally grooved nuclei, often with a single small nucleolus, are generally arranged in a double row: one row at the periphery of the cell nests and the second around the hyaline deposits. Mitoses are rare (see Media file 41-42).
Sex cord-stromal tumor with annular tubules shows complex annular tubular pattern consisting of pale cells arranged around multiple hyaline bodies (H&E, x200).
Prognosis
All Peutz–Jeghers syndrome-associated tumorlets are benign, while up to 25% of sex cord tumors with annular tubules that occur in the absence of the Peutz–Jeghers syndrome are clinically malignant. Tumors with an infiltrative growth pattern and mitotic figures beyond the usual 3-4MF/10HPF are more likely to recur or behave aggressively.1
All Peutz–Jeghers syndrome-associated tumorlets are benign, while up to 25% of sex cord tumors with annular tubules that occur in the absence of the Peutz–Jeghers syndrome are clinically malignant. Tumors with an infiltrative growth pattern and mitotic figures beyond the usual 3-4MF/10HPF are more likely to recur or behave aggressively.1
Differentials
Gonadoblastoma may simulate SCTAT but contains intratubular germ cells. A history of gonadal dysgenesis may also be present.
Adult granulosa cell tumor and Sertoli cell tumor may also resemble SCTAT, but typical areas of granulosa cell tumor or Sertoli cell tumor are almost always present on further sectioning.
Gynandroblastoma
The term gynandroblastoma should be used only for those tumors that show admixture of well-differentiated Sertoli cell and granulosa cell components with the second cell population comprising at least 10% of the lesion.69
Gynandroblastoma is very rare and occurs in a wide age range but more commonly in young adults.
Gross findings
Gynandroblastomas are variable in size with predominantly solid cut section.
Microscopic picture
The ovarian elements are seen as nests of mature granulosa cells in which Call–Exner bodies may be found. Male or testicular components are in the form of well-formed hollow tubules lined by typical Sertoli cells or Leydig cells containing Reinke crystals (see Media file 43). Several cases of gynandroblastoma have been reported in which the ovarian-type component resembled juvenile granulosa cell tumor.70,71,72
Gross findings
Gynandroblastomas are variable in size with predominantly solid cut section.
Microscopic picture
The ovarian elements are seen as nests of mature granulosa cells in which Call–Exner bodies may be found. Male or testicular components are in the form of well-formed hollow tubules lined by typical Sertoli cells or Leydig cells containing Reinke crystals (see Media file 43). Several cases of gynandroblastoma have been reported in which the ovarian-type component resembled juvenile granulosa cell tumor.70,71,72
Gynandroblastoma shows granulosa cell with well-formed Call-Exner body on the left admixed with well-differentiated Sertoli cell tubules on the right.
Adult granulosa cell tumor with minor Sertoliform areas and Sertoli cell tumor with minor foci of granulosa cell differentiation are distinguished from gynandroblastoma by the presence of at least 10% of the secondary component in gynandroblastoma. In addition, both components should be well differentiated.
Steroid Cell Tumors
Definition
Steroid cell tumors are tumors composed entirely or predominantly of cells resembling steroid hormone secreting cells.1
Steroid Cell Tumors, not Otherwise Specified
EpidemiologySteroid cell tumors, NOS occur most often in women of reproductive age, particularly during the third and fourth decades, and rarely in postmenopausal women or children. They are clinically androgenic in 40% of cases and regularly secrete androstenedione, α-hydroxyprogesterone, and testosterone.73,74
Gross findings
Steroid cell tumors are usually well circumscribed, unilateral, and solid with cystification due to degeneration and hemorrhage. They vary greatly in size from 0.5 to 45 cm in diameter. The cut surface, which bulges in the fresh state, is frequently lobulated and ranges in color from bright yellow through red-brown to dark green-brown.75
Microscopic findings
Steroid cell tumors are well demarcated from the surrounding compressed ovarian stroma. They usually have an organoid pattern common to many steroid-producing tumors, consisting of rounded or polygonal vacuolated cells arranged in nests or columns and separated by a rich network of capillaries and vascular sinusoids. The tumor cell cytoplasm is moderate to abundant in amount and varies from granular and eosinophilic to clear (see Media file 44-45). Reticulin fibers invest individual or small groups of cells, but interstitial fibrosis and hyalinization are rarely prominent. Nuclei tend to be small and central with effaced chromatin. Varying degrees of nuclear pleomorphism and mitotic activity have been observed, but a definitive diagnosis of malignancy can only really be made by the presence of local invasion.76
Steroid cell tumors are well demarcated from the surrounding compressed ovarian stroma. They usually have an organoid pattern common to many steroid-producing tumors, consisting of rounded or polygonal vacuolated cells arranged in nests or columns and separated by a rich network of capillaries and vascular sinusoids. The tumor cell cytoplasm is moderate to abundant in amount and varies from granular and eosinophilic to clear (see Media file 44-45). Reticulin fibers invest individual or small groups of cells, but interstitial fibrosis and hyalinization are rarely prominent. Nuclei tend to be small and central with effaced chromatin. Varying degrees of nuclear pleomorphism and mitotic activity have been observed, but a definitive diagnosis of malignancy can only really be made by the presence of local invasion.76
Steroid cell tumor shows diffuse proliferation of polygonal-to-rounded cells with markedly vacuolated cytoplasm, distinct cell borders, and central nuclei. Other steroid cell tumors feature more eosinophilic cytoplasm (H&E, x200).
Prognosis and predictive factors
About 30% of cases are clinically malignant and have an extraovarian spread of tumor at the time of operation. Pathological findings associated with malignant behavior include 2 or more mitotic figures/10 high power fields, necrosis, hemorrhage, diameter greater than 7 cm, moderate-to-marked nuclear atypia. However, occasional tumors that appear cytologically bland may be clinically malignant.
Differential diagnosis
Stromal luteoma is confined to the ovarian stroma and associated with stromal hyperthecosis.
Leydig cell tumors are localized to the ovarian hilus and contain Reinke crystals.
Pregnancy luteomas are more commonly multiple (approximately 50%) and bilateral (approximately 30%). Pregnancy luteoma is usually discovered in patients at the time of caesarean section and may additionally demonstrate foci of regressive or degenerative change.
Lipid-rich Sertoli cell tumor may be misinterpreted as steroid cell tumor; the presence of more typical areas of Sertoli cell tumor allows the correct diagnosis.
Lipid-rich Sertoli cell tumor may be misinterpreted as steroid cell tumor; the presence of more typical areas of Sertoli cell tumor allows the correct diagnosis.
The differential diagnosis of steroid cell tumors also includes oxyphilic variants of other ovarian tumors (eg, oxyphilic clear cell carcinomas, oxyphilic endometrioid carcinomas, metastatic malignant melanoma, metastatic renal cell carcinoma, and metastatic hepatocellular carcinoma). The identification of the typical morphological and immunohistochemical patterns of the previously mentioned tumors (eg, cytokeratin 7 and EMA in clear cell and endometrioid carcinoma; S100 protein and other melanocytic markers in melanoma; CD10 in renal cell carcinoma, and HEPAR1 in hepatocellular carcinoma) usually help in the differential diagnosis.
Stromal Luteoma
Definition ;
Stromal luteoma are defined as small steroid cell tumors that are confined to the ovarian stroma and don't have crystals of Reinke.
Stromal luteoma usually occurs in postmenopausal women and is associated with estrogenic effects commonly in the form of abnormal vaginal bleeding. Rarely, androgenic manifestations may be present.
Gross findings
These tumors are usually unilateral, small (rarely exceed 3 cm), circumscribed, gray-white, or yellow masses.1
Stromal luteoma are defined as small steroid cell tumors that are confined to the ovarian stroma and don't have crystals of Reinke.
Stromal luteoma usually occurs in postmenopausal women and is associated with estrogenic effects commonly in the form of abnormal vaginal bleeding. Rarely, androgenic manifestations may be present.
Gross findings
These tumors are usually unilateral, small (rarely exceed 3 cm), circumscribed, gray-white, or yellow masses.1
Microscopic findings
Stromal luteoma appears as nodules of luteinized stromal cells that may be arranged diffusely or in nests and cords. The cytoplasm is pale or eosinophilic, the nuclei are bland, and mitoses are rare. Foci of degenerative change, lipochrome pigment, and hyalinized stroma may be present. Stromal luteomas are usually associated with stromal hyperthecosis in the same or contralateral ovary in 90% of cases.
Stromal luteoma appears as nodules of luteinized stromal cells that may be arranged diffusely or in nests and cords. The cytoplasm is pale or eosinophilic, the nuclei are bland, and mitoses are rare. Foci of degenerative change, lipochrome pigment, and hyalinized stroma may be present. Stromal luteomas are usually associated with stromal hyperthecosis in the same or contralateral ovary in 90% of cases.
Differential diagnosis
Stromal hyperthecosis exhibits prominent spindle cell elements with admixed nests of lutein cells, which in some cases may form nodules (referred to as nodular hyperthecosis). In this instance, a cut off of 0.5 cm in diameter is used to distinguish large hyperthecotic nodule from stromal luteoma.19
Leydig Cell Tumors
Leydig cell tumors are rare ovarian steroid cell neoplasms composed of Leydig cell that contain Reinke crystals. Depending on their location, they are divided into hilus cell tumors and Leydig cell tumors of nonhilar type.1
Hilus Cell Tumors
Definition
A hilus cell tumor is a Leydig cell tumor of the ovary that arises in the ovarian hilus.77
A hilus cell tumor is a Leydig cell tumor of the ovary that arises in the ovarian hilus.77
Gross findings;
Leydig cell tumor, hilar cell type, are unilateral, small, mostly microscopic, yellow-to-brown, soft, fleshy, circumscribed masses in the hilar region of the ovary and adjacent mesovarium.
Microscopic findings
Leydig cell tumors are composed of closely packed sheets or solid cords of uniform, polyhedral, and eosinophilic cells. Nuclei are round, central, and vary in size. They frequently give an appearance of being unevenly distributed in the tumor with ”nuclear-rich” and ”nuclear-poor” zones, a feature regarded as almost pathognomonic of Leydig cell differentiation, even in the absence of Reinke crystals (see Media files 46-47). Mitoses are rare. Leydig cell cytoplasm is densely eosinophilic and finely granular with small lipid-containing cytoplasmic vacuoles. PAS-positive yellow-brown lipochrome pigment is seen in many cells.
Leydig cell tumor, hilar cell type, are unilateral, small, mostly microscopic, yellow-to-brown, soft, fleshy, circumscribed masses in the hilar region of the ovary and adjacent mesovarium.
Microscopic findings
Leydig cell tumors are composed of closely packed sheets or solid cords of uniform, polyhedral, and eosinophilic cells. Nuclei are round, central, and vary in size. They frequently give an appearance of being unevenly distributed in the tumor with ”nuclear-rich” and ”nuclear-poor” zones, a feature regarded as almost pathognomonic of Leydig cell differentiation, even in the absence of Reinke crystals (see Media files 46-47). Mitoses are rare. Leydig cell cytoplasm is densely eosinophilic and finely granular with small lipid-containing cytoplasmic vacuoles. PAS-positive yellow-brown lipochrome pigment is seen in many cells.
Higher magnification of Leydig cell tumor shows Leydig cells with abundant eosinophilic cytoplasm and characteristic nuclear-rich zones separated by nuclear-free zones. The arrows point to crystals of Reinke (H&E, x400).
Reinke crystals are slender rods with square or tapered ends, within an incomplete ”halo,” and best seen when stained bright red with Mallory trichrome stain. They are found in just over 50% of these tumors, but are irregularly distributed in the tumor and thus may require extensive searching to locate them. Other histological features of the tumor include hilus cell hyperplasia, association with nerve fibers, and fibrinoid necrosis of the blood vessels.
Leydig Cell Tumor, Nonhilar Type
A Leydig cell tumor originates from the ovarian stroma and contains Reinke crystals.Leydig cell tumor, nonhilar type, is composed of steroid cells containing Reinke crystals and surrounded by ovarian stroma that often shows stromal hyperthecosis, and except for their location, the clinical and pathological features of the nonhilar type is similar to that of the hilar type.1
Multimedia
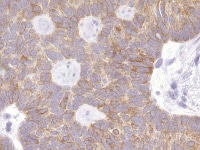 | Media file 2: Granulosa cell tumor, microfollicular pattern shows strong staining with inhibin (anti-inhibin, x400). |
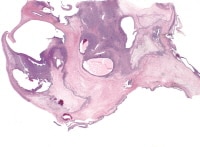 | Media file 3: Granulosa cell tumor, macrofollicular pattern (H&E, x100). |
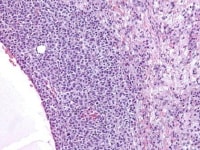 | Media file 4: Higher magnification of tumor depicted in Media file 2 shows a follicle lined by granulosa cells (H&E, x200). |
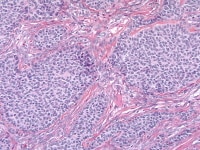 | Media file 5: Granulosa cell tumor, insular pattern (H&E, x200). |
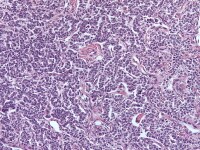 | Media file 6: Granulosa cell tumor, gyriform pattern (H&E, x200). |
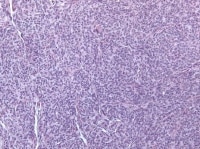 | Media file 7: Granulosa cell tumor, diffuse pattern (H&E, x100). |
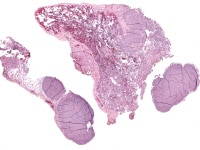 | Media file 8: Metastatic granulosa cell tumor to the lung (H&E, x5). |
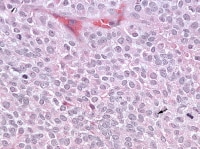 | Media file 9: Higher magnification of the metastatic granulosa cell tumor shows nodules of granulosa cells with nuclear grooves (arrow) and brisk mitotic activity (H&Ex 400). |
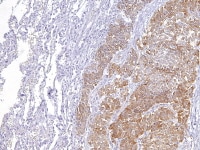 | Media file 10: Metastatic ovarian granulosa cell tumor to the lung exhibits strong positive staining for inhibin while the rest of the lung is negative (anti-inhibin, x200). |
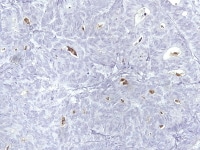 | Media file 12: Endometrioid adenocarcinoma resembling granulosa cell tumor shows negative staining for inhibin (anti-inhibin, x400). |
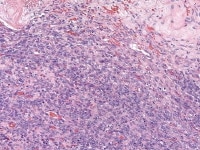 | Media file 13: Endometrioid stromal sarcoma shows diffuse proliferation of round-to-oval cells, which could be mistaken for cellular fibroma (H&E, x200). |
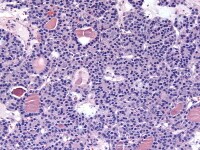 | Media file 14: Struma ovarii shows follicular and microacinar growth pattern. In some areas, the microfollicles contain dense, colloidlike material (H&E, x200). |
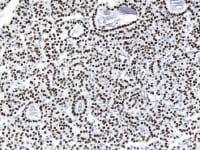 | Media file 15: Struma ovarii shows positive nuclear staining for TTF-1 (H&E, x200). |
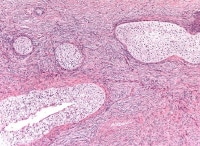 | Media file 16: Benign Brenner tumor shows sharply demarcated nests of transitional epithelial cells lying within an abundant fibrous stroma (H&E, x100). |
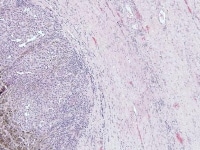 | Media file 17: A case of metastatic melanoma to the ovary shows ovary to the right infiltrated by nodule of malignant melanoma to the left with focal melanin pigment (H&E, x100). |
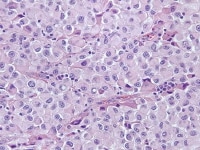 | Media file 18: Higher magnification of the metastatic melanoma shows eosinophilic cells with pleomorphic nuclei. Occasional nucleoli and cytoplasmic pseudoinclusions are present (H&E, x400). |
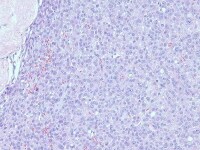 | Media file 19: Metastatic breast carcinoma shows diffuse infiltration by lobular-type epithelial cells, showing uniform nuclei with small but distinct nucleoli and eosinophilic cytoplasm (H&E x200). |
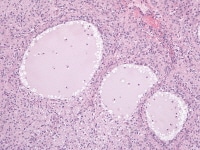 | Media file 20: Juvenile granulosa cell tumor shows round-to-oval follicles that contain eosinophilic secretion; the follicles are separated by luteinized granulosa cells (H&E, x200). |
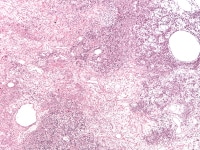 | Media file 21: Juvenile granulosa cell tumor. Follicles of varying sizes and shapes are separated by cellular areas (H&E, x40). |
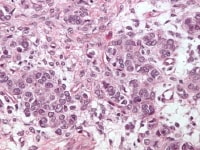 | Media file 22: Juvenile granulosa cell tumor. The cells have abundant eosinophilic cytoplasm; their nuclei are hyperchromatic and lack grooves (H&E, x400). |
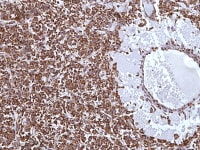 | Media file 23: Juvenile granulosa cell tumor demonstrating strong staining for inhibin (anti-inhibin, x200). |
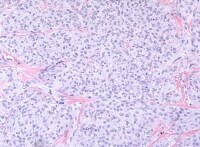 | Media file 25: Thecoma. The tumor cells have abundant pale cytoplasm. Hyaline plaques are conspicuous (H&E, x200). |
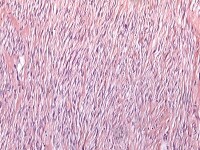 | Media file 26: Fibroma. The cells have small, spindle-shaped nuclei lacking atypia or mitotic activity (H&E, x100). |
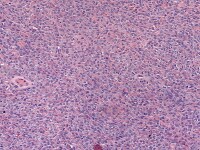 | Media file 27: Cellular fibroma. The tumor is cellular but shows no cytological atypia (H&E, x100). |
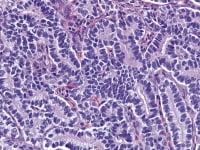 | Media file 28: Sertoli cell tumor shows closely packed hollow and solid tubules lined by well-differentiated cuboidal-to-columnar epithelial cells. Leydig cell are absent (H&E, x400). |
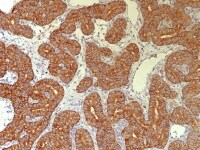 | Media file 29: Well-differentiated Sertoli cell tumor with arrow that shows strong cytoplasmic staining for inhibin in both Sertoli and Leydig cells (anti-inhibin, x200). |
 | Media file 30: Low magnification of Sertoli-Leydig cell tumor of intermediate differentiation shows cellular lobules separated by stroma (H&E, x40). |
 | Media file 32: Poorly differentiated Sertoli-Leydig cell tumor shows diffuse solid proliferation of immature Sertoli cells (H&E, x100). |
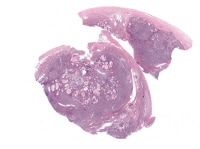 | Media file 34: Moderately differentiated Sertoli-Leydig cell tumor with heterologous elements. Note the presence of the epithelial glands in the middle of the field (H&E, x5). |
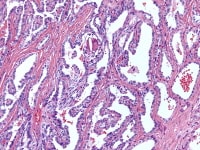 | Media file 37: Retiform Sertoli-Leydig cell tumor shows admixtures of branching retiform tubules and papillary areas with prominent hyalinized cores (H&E, x200). |
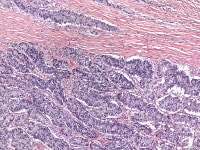 | Media file 38: Sertoliform endometrioid carcinoma composed of compact solid tubules (H&E, x100). |
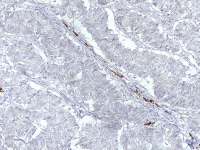 | Media file 39: Sertoliform endometrioid carcinoma demonstrating negative staining for inhibin; note positive staining of the stromal cells, indicated by the arrow (anti-inhibin, x400). |
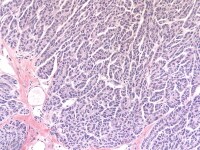 | Media file 40: Ovarian tumor of probable wolffian origin composed of uniform epithelial cells arranged in irregular tubules, nests, and cords (H&E, x100). |
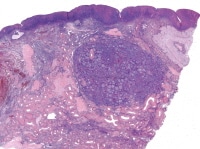 | Media file 41: Sex cord tumorlet with annular tubules detected incidentally within the ovary (H&E, x10). |
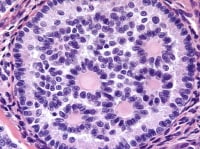 | Media file 42: Sex cord-stromal tumor with annular tubules shows complex annular tubular pattern consisting of pale cells arranged around multiple hyaline bodies (H&E, x200). |
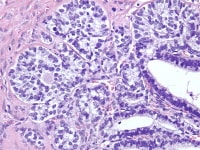 | Media file 43: Gynandroblastoma shows granulosa cell with well-formed Call-Exner body on the left admixed with well-differentiated Sertoli cell tubules on the right. |
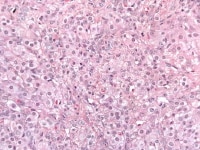 | Media file 45: Steroid cell tumor shows steroid cells with abundant eosinophilic cytoplasm (H&E, x400). |
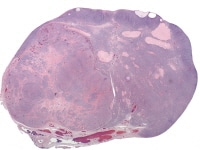 | Media file 46: Leydig cell tumor (H&E, x5). |
Keywords
ovarian tumors, sex cord-stromal tumors, granulosa cell tumor, Call-Exner bodies, thecoma, fibroma, sclerosing stromal tumor, signet ring cell stromal tumor, Sertoli-stromal cell tumor, Sertoli cell tumors, Sertoli-Leydig cell tumor, androblastoma, sex cord tumor with annular tubules, gynandroblastoma, steroid cell tumor, stromal luteoma, Leydig cell tumor, inhibin, calretininvvv




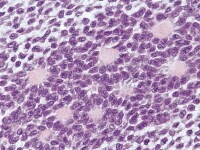
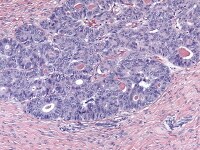
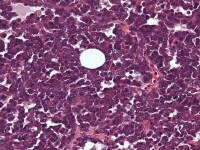
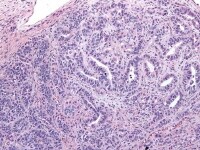
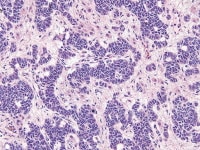
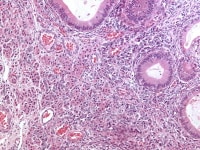
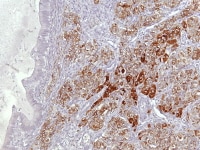
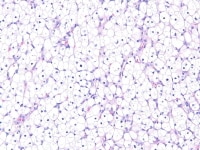
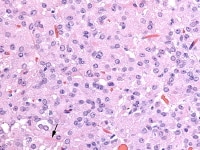

0 comments:
Post a Comment