Therapy-Related Acute Myeloid Leukemia, Myelodysplastic Syndromes, and Myelodysplastic/Myeloproliferative Neoplasms
Overview
Therapeutic advances have dramatically influenced the clinical course of numerous malignant, reactive, and autoimmune disorders. However, the therapies themselves can be mutagenic. Therapy-related myeloid neoplasms (t-MN) are a heterogeneous group of clonal hematopoietic stem cell disorders that are directly related to previous cytotoxic chemotherapy and/or radiation therapy. Two predominant and clinically significant types of t-MN have been defined, those arising after treatment with alkylating chemotherapy and/or radiation therapy and those arising after therapy with topoisomerase II inhibitors. Other drug classes (ie, antimetabolites/immunosuppressants) have been implicated in the development of these neoplasms, but in these cases, the clinical course is less distinct.
Disorders
Therapy-related myeloid neoplasms (t-MN) include the following:
- Myelodysplastic syndromes (t-MDS)
- Acute myeloid leukemia (t-AML)
- Myelodysplastic/myeloproliferative neoplasms (t-MDS/MPN)
Definition
Therapy-related myeloid neoplasms (t-MN) are defined by the World Health Organization as clonal hematopoietic stem cell disorders related to previous exposure to chemotherapy and/or radiation therapy.1 The t-MN category represents a heterogeneous group of myeloid neoplasms that share diagnostic features of conventionally defined myeloid malignancies, including myelodysplastic syndromes (MDS), acute myeloid leukemia (AML), andmyelodysplastic/myeloproliferative neoplasms (MDS/MPN). However, the therapy-related neoplasms progress quickly regardless of their morphologic appearance at presentation and are considered to be a single diagnostic entity.
On the basis of clinical behavior and morphologic features, 2 groups of t-MN have been defined; these 2 groups are distinguished principally on the basis of the nature of cytotoxic therapy. Cases of t-MN that arise following therapy with alkylating agents (eg, cyclophosphamide, chlorambucil, cisplatin) and/or ionizing radiation have a relatively long latency period (5-10 years) after primary exposure. On bone marrow aspiration, the morphologic features are those of myelodysplasia. Patients who are exposed to topoisomerase II inhibitors (eg, etoposide, doxorubicin) tend to present with frank leukemia within 1 year of the time primary therapy was initiated.
On the basis of clinical behavior and morphologic features, 2 groups of t-MN have been defined; these 2 groups are distinguished principally on the basis of the nature of cytotoxic therapy. Cases of t-MN that arise following therapy with alkylating agents (eg, cyclophosphamide, chlorambucil, cisplatin) and/or ionizing radiation have a relatively long latency period (5-10 years) after primary exposure. On bone marrow aspiration, the morphologic features are those of myelodysplasia. Patients who are exposed to topoisomerase II inhibitors (eg, etoposide, doxorubicin) tend to present with frank leukemia within 1 year of the time primary therapy was initiated.
Epidemiology
The incidence of t-MN is dependent on the type, dose, and intensity of therapeutic intervention and on the nature of the underlying primary malignancy/disease process. All age groups are affected; however, the risk of developing a secondary myeloid neoplasms following alkylating chemotherapy or radiation therapy seems to increase with age. The risk of developing t-MN dramatically decreases after 10 years.2
An extensive review and analysis of previously published data highlighted vague trends, with the greatest likelihood of developing t-MN following treatment of hematopoietic malignancies.3 However, significant variability between studies was also demonstrated, and the precise incidence is not clear. Approximately 30% of t-MN cases involve patients treated for non-neoplastic disorders and those treated with high-dose chemotherapy followed by autologous stem celltransplantation.4,5 Significantly, cases of t-MN representapproximately 10-30% of all confirmed cases of MDS, AML, and MDS/MPN.3,4
The heritable risk factors predisposing to the development of t-MN are the topic of intense study. Defects in the RAS-BRAF signal-transduction pathway, point mutations of AML1 and p53, and polymorphisms affecting drug metabolism have been implicated.6,7
Clinical Features
The clinical presentation is largely dependent on the nature of the antecedent therapeutic regimen. Patients treated with alkylating agents and/or radiation therapy typically present 5-10 years after exposure; the latency period for patients treated with topoisomerase inhibitors is on the order of 1-5 years. Despite the archetypal and often distinguishing morphologic features of these 2 groups, the clinical presentations are nonspecific and are related to the degree of bone marrow failure. Clinical features are generally related to the corresponding cytopenia (eg, bleeding with thrombocytopenia; fatigue and exercise intolerance with anemia; fever and infection with neutropenia).
Morphologic Features
Most commonly, the morphologic features of alkylating agent/radiotherapy–related t-MN are those of myelodysplasia, in particular, refractory cytopenia with multilineage dysplasia (RCMD). Evaluation of the peripheral blood smear demonstrates 1 or more cytopenias, often including a macrocytic anemia. Platelets may show nonspecific variation in size; giant forms may be present. Dysplasia of the granulocyte lineage may be assessed on the peripheral blood smear; hypolobated nuclei and hypogranularity are common features.
The bone marrow is most frequently normocellular to hypercellular with a variable blast percentage. The erythroid series may show the full breadth of dysplasia, including nuclear and cytoplasmic atypia (see Media file 1), as well as megaloblastoid features. Ring sideroblasts are often present and may be identified with iron stains (eg, Prussian blue) (see Media file 2). Megakaryocyte dysplasia is manifest as hypolobate or hyperlobate forms,megakaryocytes with nuclear segregation (see Media file 3), and/or microcytic forms with abnormal paratrabecular localization and clustering (see Media files 4-5). As with peripheral blood, the bone marrow granulocyte lineage may show variation and abnormalities of granulation and nuclear hypolobation ofthe mature forms (ie, pseudo–Pelger-Huet anomaly) (see Media file 6).
The bone marrow is most frequently normocellular to hypercellular with a variable blast percentage. The erythroid series may show the full breadth of dysplasia, including nuclear and cytoplasmic atypia (see Media file 1), as well as megaloblastoid features. Ring sideroblasts are often present and may be identified with iron stains (eg, Prussian blue) (see Media file 2). Megakaryocyte dysplasia is manifest as hypolobate or hyperlobate forms,megakaryocytes with nuclear segregation (see Media file 3), and/or microcytic forms with abnormal paratrabecular localization and clustering (see Media files 4-5). As with peripheral blood, the bone marrow granulocyte lineage may show variation and abnormalities of granulation and nuclear hypolobation ofthe mature forms (ie, pseudo–Pelger-Huet anomaly) (see Media file 6).
Pseudo–Pelger-Huet cell: granulocyte with bilobed nucleus attached by thin band of chromatin/nuclear membrane.
Cases related to treatment with topoisomerase II inhibitors typically present as overt acute myeloid leukemia (seeMedia file 7). Morphologic features are not unique in therapy-related cases. Although monocytic and monoblastic differentiation is often present, the appearance may be that of de novo cases, including those with recurrent cytogenetic abnormalities. Cases of therapy-related acute lymphoblastic leukemia (ALL) have also been described.8
Acute myeloid leukemia from a patient who presented 10 months after therapy with topoisomerase II inhibitor.
Importantly, the 2 treatment-related groups cannot always be distinguished on the basis of histologic findings; a complete clinical history, in addition to cytogenetic studies, is necessary to accurately diagnose and prognosticate these neoplasms. The morphologic findings are not entirely specific and are rarely distinguishable from those of de novo cases of AML, MDS, or MDS/MPN. In contrast to de novo myeloid neoplasms, however, there is no significant clinical or prognostic benefit to further subclassification.9
Immunophenotypic Features and Methods
Immunophenotypic studies are not used to distinguish t-MN from de novo cases of myeloid neoplasms but rather to clarify abnormal populations. The myeloblasts are characteristically CD34-positive; their distribution and burden may be demonstrated with CD34 immunohistochemical studies.
Flow cytometric analysis may be helpful in assessing the proportion of myeloid blasts, as well as aberrant antigenic expression. Aberrant expression of CD7 (a T-cell–associated antigen), CD56 (a natural killer (NK)-cell–associated antigen), and/or CD19 (a B-cell–associated antigen) may be seen in t-MN blasts. The relevance of such findings and of associated cytogenetic changes is similar to that in de novo cases. Immunophenotypic maturation patterns of the myeloid and erythroid lineages may also be evaluated; they may be particularly helpful when morphologic and cytogenetic evaluations of the bone marrow are inconclusive.10,11
Flow cytometric analysis may be helpful in assessing the proportion of myeloid blasts, as well as aberrant antigenic expression. Aberrant expression of CD7 (a T-cell–associated antigen), CD56 (a natural killer (NK)-cell–associated antigen), and/or CD19 (a B-cell–associated antigen) may be seen in t-MN blasts. The relevance of such findings and of associated cytogenetic changes is similar to that in de novo cases. Immunophenotypic maturation patterns of the myeloid and erythroid lineages may also be evaluated; they may be particularly helpful when morphologic and cytogenetic evaluations of the bone marrow are inconclusive.10,11
Molecular/Genetic Features and Methods
Cytogenetic abnormalities are present in the majority of cases of t-MN and are related to the therapeutic regimen.4,5 Conventional cytogenetic analysis (ie, karyotype) and fluorescence in situ hybridization (FISH) studies for specific abnormalities may be helpful in the diagnosis and prognostication of disease.
Alkylating agents and radiation therapy
This cohort characteristically shows unbalanced translocations and partial or complete loss of chromosomes 7 and/or 5. Monosomy 7 is the most common finding associated with alkylating agents; loss of the long arm of chromosome 5 (5q-) is the most common finding associated with ionizing radiation. Additional chromosomal abnormalities are common, yielding a complex karyotype.4
Topoisomerase II inhibitors
Cases arising after use of topoisomerase II inhibitors are associated with a short latency to presentation. Balanced translocations are also seen in these cases. Frequent rearrangements of 11q23(MLL) and 21q22(AML1) are identified. Balanced translocations seen in de novo cases of AML are also present, including t(15;17)(q22;q21), inv(16)(p13.q22), and t(8;21)(q22;q22), although they are less common than in de novo cases.4,5
Alkylating agents and radiation therapy
This cohort characteristically shows unbalanced translocations and partial or complete loss of chromosomes 7 and/or 5. Monosomy 7 is the most common finding associated with alkylating agents; loss of the long arm of chromosome 5 (5q-) is the most common finding associated with ionizing radiation. Additional chromosomal abnormalities are common, yielding a complex karyotype.4
Topoisomerase II inhibitors
Cases arising after use of topoisomerase II inhibitors are associated with a short latency to presentation. Balanced translocations are also seen in these cases. Frequent rearrangements of 11q23(MLL) and 21q22(AML1) are identified. Balanced translocations seen in de novo cases of AML are also present, including t(15;17)(q22;q21), inv(16)(p13.q22), and t(8;21)(q22;q22), although they are less common than in de novo cases.4,5
Prognosis and Predictive Factors
Although t-MDS, t-MDS/MPN, and t-AML have morphologically disparate phases, rapid progression and shortened survival are common in all cases, irrespective of subclassification.9 Overall survival is poor (<10% at 5 years).1,12
As with other myeloid neoplasms, the cytogenetic features of t-MN are predictive of outcome. However, despite the morphologic and cytogenetic similarities of some t-MN and de novo myeloid neoplasms, therapy-induced disease typically carries a worse prognosis than its de novo counterpart.13 Balanced translocations are associated with a relatively better prognosis, whereas loss of the entire or partial chromosomes 5 or 7 is associated with a particularly dismal prognosis.14
As seen in de novo cases of AML, favorable cytogenetic findings, including t(15;17)(q22;q12) and inv(16)(p13.1q22), confer a relatively better prognosis; however, this is not universal. A recent study comparing t(8;21) t-AML to de novo t(8;21) AML demonstrated that despite virtually indistinguishable morphologic, immunophenotypic, and cytogenetic features, the median overall survival was dramatically worse in therapy-related cases.15
As with other myeloid neoplasms, the cytogenetic features of t-MN are predictive of outcome. However, despite the morphologic and cytogenetic similarities of some t-MN and de novo myeloid neoplasms, therapy-induced disease typically carries a worse prognosis than its de novo counterpart.13 Balanced translocations are associated with a relatively better prognosis, whereas loss of the entire or partial chromosomes 5 or 7 is associated with a particularly dismal prognosis.14
As seen in de novo cases of AML, favorable cytogenetic findings, including t(15;17)(q22;q12) and inv(16)(p13.1q22), confer a relatively better prognosis; however, this is not universal. A recent study comparing t(8;21) t-AML to de novo t(8;21) AML demonstrated that despite virtually indistinguishable morphologic, immunophenotypic, and cytogenetic features, the median overall survival was dramatically worse in therapy-related cases.15
DIFFERENTIALS
Multimedia
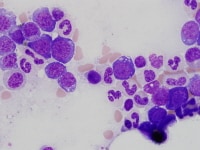 | Media file 1: Nucleated erythroid precursors with cytoplasmic vacuolization. |
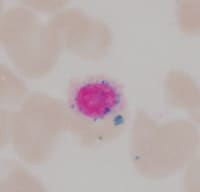 | Media file 2: Ring sideroblast. |
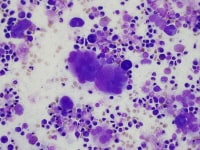 | Media file 3: Abnormal megakaryocytes with segregated nuclei. |
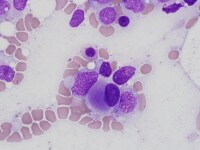 | Media file 4: Micromegakaryocyte. |
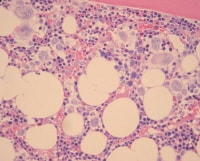 | Media file 5: Bone marrow biopsy specimen showing erythroid hyperplasia and clusters of abnormal megakaryocytes. |
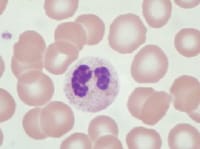 | Media file 6: Pseudo–Pelger-Huet cell: granulocyte with bilobed nucleus attached by thin band of chromatin/nuclear membrane. |
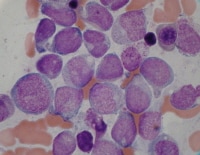 | Media file 7: Acute myeloid leukemia from a patient who presented 10 months after therapy with topoisomerase II inhibitor. |
Keywords
therapy-related acute myeloid leukemia, therapy-related myeloid neoplasms, myelodysplastic syndrome, myelodysplastic/myeloproliferative neoplasms, t-MN, t-MDS, t-AML, t-MDS/MPN, alkylating agents, topoisomerase II inhibitors





0 comments:
Post a Comment